Abstract
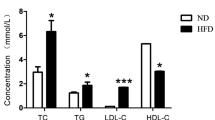
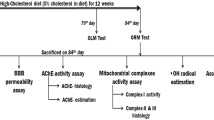
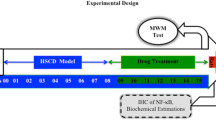
Alzheimer's disease (AD) is the most common form of dementia that progressively disrupts neurocognitive function, which has neither cure nor effective treatment. Hypercholesterolemia might be involved in brain alterations that could evolve into AD. The present study aims to evaluate the potential of omega-3, Co-enzyme Q10 (Co-Q10), as well as their combination in ameliorating hypercholesterolemia-initiated AD-like disease. We adapted a hypercholesterolemic (HC) rat model, a model of oxidative stress-mediated neurodegeneration, to study AD-like pathology. Hypercholesterolemia resulted in increased lipid peroxidation coupled with declined nitric oxide production, reduced glutathione levels, and decreased antioxidant activities of glutathione-s-transferase (GST) and glutathione peroxidase (GSH-Px) in the brain. Moreover, hypercholesterolemia resulted in decreased acetylcholine (ACh) levels and increased acetylcholine-esterase (AChE) activity, along with an increment of tumor necrosis factor and amyloid-β 42. Behaviorally, HC-rats demonstrated depressive-like behavior and declined memory. Treatment of HC-rats with omega-3 and Co-Q10 (alone or in combination) alleviated the brain oxidative stress and inflammation, regulated cholinergic functioning, and enhanced the functional outcome. These findings were verified by the histopathological investigation of brain tissues. This neuroprotective potential of omega-3 and Co-Q10 was achieved through anti-oxidative, anti-inflammatory, anti-amyloidogenic, pro-cholinergic, and memory-enhancing activities against HC-induced AD-like disease; suggesting that they may be useful as prophylactic and therapeutic agents against the neurotoxic effects of hypercholesterolemia.








Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer's disease
- AChE:
-
Acetylcholine-esterase
- TNF-α:
-
Tumor necrosis factor-α
- Aβ-42:
-
Amyloid-β 42
- ACh:
-
Acetylcholine
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- GST:
-
Glutathione-s-transferase
- GSH-Px:
-
Glutathione peroxidase
References
Alzheimer Association (2016) Alzheimer’s disease facts and figures. Alzheimer’s Dement 12:1–80. https://doi.org/10.1016/j.jalz.2016.03.001
Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C (2014) Potential for primaryprevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 13:819–828
Petrov D, Pedros I, Artiach G, Sureda FX, Barroso E, Pallas M et al (2015) High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiencies contribute to Alzheimer disease pathology in rodents. Biochim Biophys Acta 1852(9):1687–1699
Shefer G, Marcus Y, Stern N (2013) Is obesity a brain disease? Neurosci Biobehav Rev 37(10 Pt 2):2489–2503
Bane TJ, Cole C (2015) Prevention of Alzheimer disease: the roles of nutrition and primary care. Nurse Pract 40(5):30–36
Miyake Y, Tanaka K, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y et al (2010) Case-control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J Neurol Sci 293(1–2):82–86
Parrott MD, Greenwood CE (2007) Dietary influences on cognitive function with aging: from high-fat diets to healthful eating. Ann N Y Acad Sci 1114:389–397
Matsuda M, Shimomura I (2013) Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract 7(5):e330–e341
Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, Hakkarainen A, Lundbom J et al (2015) Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 64(9):3135–3145
Sandoval-Salazar C, Ramirez-Emiliano J, Trejo-Bahena A, Oviedo-Solis CI, Solis-Ortiz MS (2016) A high-fat diet decreases GABA concentration in the frontal cortex and hippocampus of rats. Biol Res 49:15–20
Loffler T, Flunkert S, Temmel M, Hutter-Paier B (2016) Decreased plasma abeta in hyperlipidemic APPSL transgenic mice is associated with BBB dysfunction. Front Neurosci 10:232
van de Haar HJ, Jansen JF, van Osch MJ, van Buchem MA, Muller M, Wong SM, Hofman PA, Burgmans S, Verhey FR, Backes WH (2016) Neurovascular unit impairment in early Alzheimer's disease measured with magnetic resonance imaging. Neurobiol Aging 45:190–196
Bleckwenn M, Kleineidam L, Wagner M, Jessen F, Weyerer S, Werle J, Scherer M (2017) Impact of coronary heart disease on cognitive decline in Alzheimer's disease: a prospective longitudinal cohort study in primary care. Br J Gen Pract 67(655):e111–e117. https://doi.org/10.3399/bjgp16X688813
Silbert BS, Scott DA, Evered LA, Lewis MS, Maruff PT (2007) Preexisting cognitive impairment in patients scheduled for elective coronary artery bypass graft surgery. Anesth Analg 104(5):1023–1028. tables of contents. https://doi.org/10.1213/01.ane.0000263285.03361.3a
Qiang F, Lee BJ, Lee W, Han HK (2009) Pharmacokinetic drug interaction between fexofenadine and fluvastatin mediated by organic anion-transporting polypeptides in rats. Eur J Pharm Sci 37(3–4):413–417
Cremonini AL, Caffa I, Cea M, Nencioni A, Odetti P, Monacelli F. (2019) Nutrients in the prevention of Alzheimer’s disease. Oxid Med Cell Longev 2019
Villalba JM, Parrado C, Santos-Gonzalez M, Alcain FJ (2010) Therapeutic use of coenzyme Q 10 and coenzyme Q 10 -related compounds and formulations. Expert Opin Investig Drugs 19(4):535–554
Johnson AR, Milner JJ, Makowski L (2012) The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev 249(1):218–238
Gow RV, Hibbeln JR (2014) Omega-3 fatty acid and nutrient deficits in adverse neurodevelopment and childhood behaviors. Child Adol Psych Clin 23(3):555–590. https://doi.org/10.1016/j.chc.2014.02.002
Innis SM (2008) Dietary omega 3 fatty acids and the developing brain. Brain Res 1237:35–43
Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 233(6):674–688
Brenna JT, Diau GY (2007) The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition, prostaglandins. Leukot Essent Fatty Acids 77:247–250
Agrawal R, Gomez-Pinilla F (2012) ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signaling and cognition. J Physiol 590(Pt 10):2485–2499
Moreira JD, Knorr L, Ganzella M et al (2010) Omega-3 fatty acids deprivation affects ontogeny of glutamatergic synapses in rats: relevance for behavior alterations. Neurochem Int 56(6–7):753–759
Akbar M, Calderon F, Wen Z, Kim HY (2005) Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA 102(31):10858–10863
Bookstaver D, Nancy P, Pharm B, Hatzigeorgiou C (2012) Effect of Coenzyme Q10 supplementation on statin-Induced myalgias. Am J Cardiol 110:526–529
Bentinger M, Brismar K, Dallner G (2007) The antioxidant role of coenzyme Q. Mitochondrion S7:S41–S50
Wyman M, Leonard M, Morledge T (2010) Coenzyme Q10: a therapy for hypertension and statin-induced myalgia? Cleve Clin J Med 77:435–442
Ernster L, Dallner G (1995) Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta 1271(1):195–204
Adaramoye O, Akinatyo O, Achen J, Michel A (2008) Lipid lowering effects of methanolic extracts of Vernonia anygdalina leaves in rats fed on high cholesterol diet. Vasc Health Risk Manag 4(1):235–241
Lakhwani LALIT, Tongia SK, Pal VS, Agrawal RP, Nyati PREM, Phadnis PRADEEP (2007) Omega-3 fatty acids have antidepressant activity in forced swimming test in Wistar rats. Acta Pol Pharm 64(3):271–276
Coldiron AD, Sanders RA, Watkins JB (2002) Effects of combined quercetin and coenzyme Q(10) treatment on oxidative stress in normal and diabetic rats. J Biochem Mol Toxicol 16:197–202
Porsolt RD, Bertin A, Jalfre M (1978) Behavioural despair in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol 51:291–294
Deacon RMJ, Rawlins JNP (2006) T-maze alternation in the rodent. Nat Protoc 1:7–12. https://doi.org/10.1038/nprot
Ohkawa H et al (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Beutler E, Duron O, Kelly BM (1963) An improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Montgomery HAC, Dymock JF (1961) The determination of nitrate in water. Analyst 86:414–416
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Bio Chem 249(22):7130–7139
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clinic Med 70(1):158–169
Selkoe DJ (1994) Normal and abnormal biology of the beta-amyloid precursor protein. Ann Rev Neurosc 17(1):489–517
Banchroft TDJD, Stevens A (1996) Theory and practice of histological techniques, 4th edn. Churchil Livingstone, New York
Loera-Valencia R, Goikolea J, Parrado-Fernandez C, Merino-Serrais P, Maioli S (2019) Alterations in cholesterol metabolism as a risk factor for developing Alzheimer's disease: potential novel targets for treatment. J Steroid Biochem.
Freeman LR, Zhang L, Nair A, Dasuri K, Francis J, Fernandez-Kim SO, Bruce-Keller AJ, Keller JN (2013) Obesity increases cerebrocortical reactive oxygen species and impairs brain function. Free Radic Biol Med 56:226–233
Greenwood CE, Winocur G (1996) Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake. Behav Neurosci 110(3):451–459
Abbasnejad Z, Nasseri B, Zardooz H, Ghasemi R (2019) Time-course study of high fat diet induced alterations in spatial memory, hippocampal JNK, P38, ERK and Akt activity. Metab brain dis 34(2):659–673
Lavin DN, Joesting JJ, Chiu GS, Moon ML, Meng J, Dilger RN, Freund GG (2011) Fasting induces an anti-inflammatory effect on the neuroimmune system which a high-fat diet prevents. Obesity (Silver Spring) 19(8):1586–1594
Nguyen JC, Ali SF, Kosari S, Woodman OL, Spencer SJ, Killcross AS et al (2017) Western diet chow consumption in rats induces striatal neuronal activation while reducing dopamine levels without affecting spatial memory in the radial arm maze. Front Behav Neurosci 11:22
Singh M, Kaur M, Kukreja H, Chugh R, Silakari O, Singh D (2013) Acetylcholinesterase inhibitors as Alzheimer therapy: from nerve toxins to neuroprotection. Eur J Med Chem 70:165–188. https://doi.org/10.1016/j.ejmech.2013.09.050
Singla N, Dhawan DK (2012) Regulatory role of zinc during aluminium-induced altered carbohydrate metabolism in rat brain. J Neurosci Res 90:698–705. https://doi.org/10.1002/jnr.22790
Valladolid-Acebes I, Merino B, Principato A, Fole A, Barbas C, Lorenzo MP et al (2012) High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Am J Physiol Endocrinol Metab 302(4):E396–402
Ferraz AC, Delattre AM, Almendra RG, Sonagli M, Borges C, Araujo P, Andersen ML, Tufik S, Lima MM (2011) Chronic omega-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav Brain Res 219:116–122
Park Y, Moon HJ, Kim SH (2012) N-3 polyunsaturated fatty acid consumption produces neurobiological effects associated with prevention of depression in rats after the forced swimming test. J Nutr Biochem 23:924–928
Jackson C, Barrett DW, Shumake J, Gonzales E, Gonzalez-Lima F, Lane MA (2018) Maternal omega-3 fatty acid intake during neurodevelopment does not affect pup behavior related to depression, novelty, or learning. BMC Res Notes 11(1):812
Aboul-Fotouh S (2013) Coenzyme Q10 displays antidepressant-like activity with reduction of hippocampal oxidative/nitrosative DNA damage in chronically stressed rats. Pharmacol Biochem Behav 104:105–112
Ashkani-Esfahani S, Bagheri F, Emami Y, Esmaeilzadeh E, Azarpira N, Hassanabadi N, Noorafshan A (2016) Protective effects of co-enzyme Q10 on thioacetamide-induced acute liver damage and its correlation with behavioral, biochemical, and pathological factors. Iran Red Crescent Med J 18(8)
Minami M, Kimura S, Endo T, Hamaue N, Hirafuji M, Togashi H et al (1997) Dietary docosahexaenoic acid increases cerebral acetylcholine levels and improves passive avoidance performance in stroke-prone spontaneously hypertensive rats. Pharmacol Biochem Behav 58(4):1123–1129
Choi H, Park HH, Koh SH, Choi NY, Yu HJ, Park J et al (2012) Coenzyme Q10 protects against amyloid β-induced neuronal cell death by inhibiting oxidative stress and activating the P13K pathway. Neurotox 33:85–90. https://doi.org/10.1016/j.neuro.2011.12.005
Young AJ, Johnson S, Steffens DC, Doraiswamy PM (2007) Coenzyme Q10: a review of its promise as a neuroprotectant. CNS Spectr 12:62–68
Abdin A, Hamouda H (2008) Mechanism of the neuroprotective role of coenzyme Q10 with or without L-dopa in rotenone-induced parkinsonism. Neuropharmacol 55:1340–1346
de Mello AH, Gassenferth A, de Bona Schraiber R, da Rosa Souza L, Florentino D, Danielski LG, et al (2014) Effects of omega-3 on behavioral and biochemical parameters in rats submitted to chronic mild stress. Metab Brain Dis 29(3):691–699
Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC (2014) Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosci 17(6):241–251
Ma W, Yuan L, Yu H, Xi Y, Xiao R (2014) Mitochondrial dysfunction and oxidative damage in the brain of diet-induced obese rats but not in diet-resistant rats. Life Sci 110(2):53–60
Otunola GA, Oloyede OB, Oladiji AT, Afolayan J (2014) Selected spices and their combination modulate hypercholesterolemia-induced oxidative stress in experimental rats. Bio Res 47(1):5
Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E (2012) Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci 322(1–2):254–262
Williams TI, Lynn BC, Markesbery WR, Lovell MA (2006) Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer's disease. Neurobiol Aging 27(8):1094–1099
Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT (2011) Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14(1):123–130
Dominguez C, Ruiz E, Gussinye M, Carrascosa A (1998) Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care 21(10):1736–1742
Shrag M, Mueller C, Zabel M, Crofton A, Kirsch WM, Ghribi O (2013) Oxidative stress in blood in Alzheimer's disease and mild cognitive impairment: a meta-analysis. Neurobiol Aging 59:100–110
Kawashima S, Yokoyama M (2004) Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arteriosc Thromb Vasc Bio 24(6):998–1005
Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KTS (2015) The role of the nitric oxide pathway in brain injury and its treatment—from bench to bedside. Exp neuro 263:235–243
Hossain MS, Hashimoto M, Gamoh S, Masumura S (1999) Antioxidative effects of docosahexaenoic acid in the cerebrum versus cerebellum and brainstem of aged hypercholesterolemic rats. J Neurochem 72(3):1133–1138
Stanley WC, Khairallah RJ, Dabkowski ER (2012) Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care 15:122–126
Green P, Glozman S, Yavin E (2001) Ethyl docosahexaenoate-associated decrease in fetal brain lipid peroxide production is mediated by activation of prostanoid and nitric oxide pathways. Biochim Biophys Acta 1531(1–2):156–164
de Mello AH, de Bona SR, de Souza Goldim MP, Garcez ML, Gomes ML, de Bem SG et al (2019) Omega-3 fatty acids attenuate brain alterations in high-fat diet-induced obesity model. Molecular neurobiology 56(1):513–524
Crane FL (2001) Biochemical functions of coenzyme Q10. J Am Coll Nutr 20:591–598
Wadsworth TL, Bishop JA, Pappu AS, Woltjer RL, Quinn JF (2010) Evaluation of coenzyme Q as an antioxidant strategy for Alzheimer’s disease. J Alzheimers Dis 14:225–234
Hargreaves I (2014) Coenzyme Q 10 as a therapy for mitochondrial disease. Int J Biochem Cell Biol 49:105–111. https://doi.org/10.1016/j.biocel.2014.01.020
Oguntibeju OO (2019) Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. IJPPP 11(3):45
Cao XL, Du J, Zhang Y, Yan JT, Hu XM (2015) Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation and neuronal apoptosis in MCAO/reperfusion rats. Exp Brain Res 233(10):2753–2765
Walker JM, Dixit S, Saulsberry AC, May JM, Harrison FE (2017) Reversal of high fat diet-induced obesity improves glucose tolerance, inflammatory response, β-amyloid accumulation, and cognitive decline in the APP/PSEN1 mouse model of Alzheimer's disease. Neurobiol Dis 100:87–98
Puig KL, Floden AM, Adhikari R, Golovko MY, Combs CK (2012) Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS ONE 7(1):e30378
de Lima Oliveira BC, Bellozi PMQ, Reis HJ, de Oliveira ACP (2018) Inflammation as a possible link between dyslipidemia and Alzheimer’s Disease. Neuroscience 376:127–141
Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M, Laiglesia LM, Martínez JA et al (2015) An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem 71(2):341–349
Yates CM, Calder PC, Rainger G (2014) Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther 141(3):272–282
Yan L, Xie Y, Satyanarayanan SK, Zeng H, Liu Q, Huang M, et al (2019) Omega-3 polyunsaturated fatty acids promote brain-to-blood clearance of β-Amyloid in a mouse model with Alzheimer’s disease. Brain Behav Immun
Sanoobar M, Eghtesadi S, Azimi A, Mohammad K (2013) Coenzyme Q10 supplementtion reduces oxidative stress and increases antioxidant enzyme activity in patients with relapsing–remitting multiple sclerosis. Int J Neurosci 123:776–782
de Oliveira FE, Fernandes MYSD, de Lima NMR, Neves KRT, do Carmo MRS, Lima FAV et al (2016) Neuroinflammatory response to experimental stroke is inhibited by eriodictyol. Behav Brain Res 312:321–332
Park SH, Kim JH, Choi KH, Jang YJ, Bae SS, Choi BT, Shin HK (2013) Hypercholesterolemia accelerates amyloid beta-induced cognitive deficits. Int J Mol Med 31:577–582
Maesako M, Uemura M, Tashiro Y, Sasaki K, Watanabe K, Noda Y, Ueda K, Asada-Utsugi M (2015) High fat diet enhances beta-site cleavage of amyloid precursor protein (APP) via promoting beta-site APP cleaving enzyme 1/adaptor protein 2/clathrin complex formation. PLoS ONE 10(9):e0131199
Graham LC, Harder JM, Soto I, de Vries WN, John SW, Howell GR et al (2016) Chronic consumption of a western diet induces robust glial activation in aging mice and in a mouse model of Alzheimer's disease. Sci Report 6:21568
Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L et al (2007) Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci 27(16):4385–4395
Eckert GP, Chang S, Eckmann J, Copanaki E, Hagl S, Hener U et al (2011) Liposome-incorporated DHA increases neuronal survival by enhancing non-amyloidogenic APP processing. Biochim Biophys Acta 1808(1):236–243
Dumont M, Kipiani K, Yu F, Wille E, Katz M, Calingasan NY et al (2011) Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis 27(1):211–223
Yang X, Yang Y, Li G, Wang J, Yang ES (2008) Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci 34(2):165–171
Segarra AB, Ruiz-Sanz JI, Ruiz-Larrea MB et al (2011) The profile of fatty acids in frontal cortex of rats depends on the type of fat used in the diet and correlates with neuropeptidase activities. Horm Metab Res 43(2):86–91
Monsef A, Shahidi S, Komaki A (2019) Influence of chronic coenzyme Q10 supplementation on cognitive function, learning, and memory in healthy and diabetic middle-aged rats. Neuropsychobiology 77(2):92–100
Acknowledgements
The author would like to thank reviewers for their in-depth comments that improved the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there are no competing interests.
Ethical Approval
All procedures performed in this study were in accordance with the ethical standards of the Ethical Committee of the NRC under approval number: 19–105.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibrahim Fouad, G. Combination of Omega 3 and Coenzyme Q10 Exerts Neuroprotective Potential Against Hypercholesterolemia-Induced Alzheimer's-Like Disease in Rats. Neurochem Res 45, 1142–1155 (2020). https://doi.org/10.1007/s11064-020-02996-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-02996-2