Abstract
Introduction
Opening of the blood–brain barrier (BBB) by pulsed low intensity ultrasound has been developed during the last decade and is now recognized as a safe technique to transiently and repeatedly open the BBB. This non- or minimally invasive technique allows for a targeted and uniform dispersal of a wide range of therapeutic substances throughout the brain, including immune cells and antibodies.
Methods
In this review article, we summarize pre-clinical studies that have used BBB-opening by pulsed low intensity ultrasound to enhance the delivery of immune therapeutics and effector cell populations, as well as several recent clinical studies that have been initiated. Based on this analysis, we propose immune therapeutic strategies that are most likely to benefit from this strategy. The literature review and trial data research were performed using Medline/Pubmed databases and clinical trial registry www.clinicaltrials.gov. The reference lists of all included articles were searched for additional studies.
Results
A wide range of immune therapeutic agents, including small molecular weight drugs, antibodies or NK cells, have been safely and efficiently delivered to the brain with pulsed low intensity ultrasound in preclinical models, and both tumor control and increased survival have been demonstrated in different types of brain tumor models in rodents. Ultrasound-induced BBB disruption may also stimulate innate and cellular immune responses.
Conclusions
Ultrasound BBB opening has just recently entered clinical trials with encouraging results, and the association of this strategy with immune therapeutics creates a new field of brain tumor treatment.

Similar content being viewed by others
Abbreviations
- anti-Aß:
-
Anti-amyloïd beta
- APC:
-
Antigen-presenting cell
- BBB:
-
Blood–brain barrier
- BBBD:
-
Blood–brain barrier disruption
- CNS:
-
Central nervous system
- CD:
-
Cluster of differentiation
- CED:
-
Convection-enhanced delivery
- CTL:
-
Cytolytic T cell
- DNA:
-
Deoxyribonucleic acid
- FISPION:
-
Fluorescently-labeled superparamagnetic iron oxide nanoparticles
- FUS:
-
Focused ultrasound
- Fab:
-
Fragment antigen-binding
- GFAP:
-
Glial fibrillary acidic protein
- G-CSF:
-
Granulocyte colony-stimulating factor
- HSP70:
-
Heat-shock protein 70
- HPLC:
-
High performance liquid chromatography
- HER2:
-
Human epidermal growth factor receptor-2
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- IFNγ:
-
Interferon gamma
- IL:
-
Interleukin
- IP:
-
Intraperitoneal
- MIP3α:
-
Macrophage inflammatory protein-3 alpha
- NK:
-
Natural killer
- NF-κB:
-
Nuclear factor-kappa B
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- scFv:
-
Single-chain variable fragment
- SPIO:
-
Super-paramagnetic iron oxides
- Treg:
-
Regulatory T cell
- TNFα:
-
Tumor necrosis factor alpha
- US:
-
Ultrasound
- VEGF-A:
-
Vascular endothelial growth factor A
References
Medawar PB (1948) Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol 29:58–69
Aloisi F, Ria F, Adorini L (2000) Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today 21:141–147
Hickey WF, Hsu BL, Kimura H (1991) T-lymphocyte entry into the central nervous system. J Neurosci Res 28:254–260. https://doi.org/10.1002/jnr.490280213
Ransohoff RM, Kivisakk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3:569–581. https://doi.org/10.1038/nri1130
Cserr HF, Harling-Berg CJ, Knopf PM (1992) Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol 2:269–276. https://doi.org/10.1111/j.1750-3639.1992.tb00703.x
Cserr HF, Knopf PM (1992) Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today 13:507–512. https://doi.org/10.1016/0167-5699(92)90027-5
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341. https://doi.org/10.1038/nature14432
Ledford H (2011) Engineered antibodies cross blood-brain barrier. Nat News. https://doi.org/10.1038/news.2011.319
Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, Barthel F, Cho HJ, Lin YH, Satani N, Martinez-Ledesma E, Zheng S, Chang E, Gabriel Sauve CE, Olar A, Lan ZD, Finocchiaro G, Phillips JJ, Berger MS, Gabrusiewicz KR, Wang G, Eskilsson E, Hu J, Mikkelsen T, DePinho RA, Muller F, Heimberger AB, Sulman EP, Nam DH, Verhaak RGW (2018) Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 33:152. https://doi.org/10.1016/j.ccell.2017.12.012
Wei J, Marisetty A, Schrand B, Gabrusiewicz K, Hashimoto Y, Ott M, Grami Z, Kong L-Y, Ling X, Caruso H, Zhou S, Wang YA, Fuller GN, Huse J, Gilboa E, Kang N, Huang X, Verhaak R, Li S, Heimberger AB (2019) Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest 129:137–149
Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB (2006) The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-oncology 8:261–279
Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, Woroniecka K, Elsamadicy AA, Dechant CA, Kemeny HR, Sanchez-Perez L, Cheema TA, Souders NC, Herndon JE, Coumans JV, Everitt JI, Nahed BV, Sampson JH, Gunn MD, Martuza RL, Dranoff G, Curry WT, Fecci PE (2018) Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med 24:1459–1468. https://doi.org/10.1038/s41591-018-0135-2
Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D, Hadani M, Ram Z (2004) Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg 100:472–479. https://doi.org/10.3171/jns.2004.100.3.0472
Chen W, Wu Q, Mo L, Nassi M (2013) Intra-arterial chemotherapy is not superior to intravenous chemotherapy for malignant gliomas: a systematic review and meta-analysis. Eur Neurol 70:124–132. https://doi.org/10.1159/000346580
Ashby LS, Smith KA, Stea B (2016) Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol 14:225. https://doi.org/10.1186/s12957-016-0975-5
Farra R, Sheppard NF Jr, McCabe L, Neer RM, Anderson JM, Santini JT Jr, Cima MJ, Langer R (2012) First-in-human testing of a wirelessly controlled drug delivery microchip. Sci Transl Med 4:121–122. https://doi.org/10.1126/scitranslmed.3003276
Rapoport SI, Hori M, Klatzo I (1972) Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am J Physiol 223:323–331. https://doi.org/10.1152/ajplegacy.1972.223.2.323
Black KL, Cloughesy T, Huang SC, Gobin YP, Zhou Y, Grous J, Nelson G, Farahani K, Hoh CK, Phelps M (1997) Intracarotid infusion of RMP-7, a bradykinin analog, and transport of gallium-68 ethylenediamine tetraacetic acid into human gliomas. J Neurosurg 86:603–609. https://doi.org/10.3171/jns.1997.86.4.0603
Pardridge WM (1995) Transport of small molecules through the blood-brain barrier: biology and methodology. Adv Drug Deliv Rev 15:5–36
Ozduman K, Wollmann G, Piepmeier JM, van den Pol AN (2008) Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J Neurosci 28:1882–1893. https://doi.org/10.1523/JNEUROSCI.4905-07.2008
Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA (2001) Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 220:640–646. https://doi.org/10.1148/radiol.2202001804
Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G, Asquier N, Law-Ye B, Leclercq D, Bissery A, De Rycke Y, Trosch C, Capelle L, Sanson M, Hoang-Xuan K, Dehais C, Houillier C, Laigle-Donadey F, Mathon B, Andre A, Lafon C, Chapelon JY, Delattre JY, Carpentier A (2019) Safety and feasibility of repeated and transient blood-brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin Cancer Res 25:3793–3801. https://doi.org/10.1158/1078-0432.CCR-18-3643
Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K (2004) Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol 30:979–989. https://doi.org/10.1016/j.ultrasmedbio.2004.04.010
Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N (2005) Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 24:12–20. https://doi.org/10.1016/j.neuroimage.2004.06.046
Kobus T, Vykhodtseva N, Pilatou M, Zhang Y, McDannold N (2016) Safety validation of repeated blood-brain barrier disruption using focused ultrasound. Ultrasound Med Biol 42:481–492. https://doi.org/10.1016/j.ultrasmedbio.2015.10.009
Horodyckid C, Canney M, Vignot A, Boisgard R, Drier A, Huberfeld G, Francois C, Prigent A, Santin MD, Adam C, Willer JC, Lafon C, Chapelon JY, Carpentier A (2017) Safe long-term repeated disruption of the blood-brain barrier using an implantable ultrasound device: a multiparametric study in a primate model. J Neurosurg 126:1351–1361. https://doi.org/10.3171/2016.3.JNS151635
Downs ME, Buch A, Sierra C, Karakatsani ME, Teichert T, Chen S, Konofagou EE, Ferrera VP (2015) Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS ONE 10:e0125911. https://doi.org/10.1371/journal.pone.0125911
McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS (2012) Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res 72:3652–3663. https://doi.org/10.1158/0008-5472.CAN-12-0128
Beccaria K, Canney M, Goldwirt L, Fernandez C, Piquet J, Perier MC, Lafon C, Chapelon JY, Carpentier A (2016) Ultrasound-induced opening of the blood-brain barrier to enhance temozolomide and irinotecan delivery: an experimental study in rabbits. J Neurosurg 124:1602–1610. https://doi.org/10.3171/2015.4.JNS142893
Kinoshita M, McDannold N, Jolesz FA, Hynynen K (2006) Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun 340:1085–1090. https://doi.org/10.1016/j.bbrc.2005.12.112
Hsu YH, Liu RS, Lin WL, Yuh YS, Lin SP, Wong TT (2017) Transcranial pulsed ultrasound facilitates brain uptake of laronidase in enzyme replacement therapy for Mucopolysaccharidosis type I disease. Orphanet J Rare Dis 12:109. https://doi.org/10.1186/s13023-017-0649-6
Wang F, Shi Y, Lu L, Liu L, Cai Y, Zheng H, Liu X, Yan F, Zou C, Sun C, Shi J, Lu S, Chen Y (2012) Targeted delivery of GDNF through the blood-brain barrier by MRI-guided focused ultrasound. PLoS ONE 7:e52925. https://doi.org/10.1371/journal.pone.0052925
Huang Q, Deng J, Wang F, Chen S, Liu Y, Wang Z, Wang Z, Cheng Y (2012) Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp Neurol 233:350–356. https://doi.org/10.1016/j.expneurol.2011.10.027
Alkins R, Burgess A, Ganguly M, Francia G, Kerbel R, Wels WS, Hynynen K (2013) Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res 73:1892–1899. https://doi.org/10.1158/0008-5472.CAN-12-2609
Ting CY, Fan CH, Liu HL, Huang CY, Hsieh HY, Yen TC, Wei KC, Yeh CK (2012) Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 33:704–712. https://doi.org/10.1016/j.biomaterials.2011.09.096
Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K (2012) Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol 38:1716–1725. https://doi.org/10.1016/j.ultrasmedbio.2012.04.015
Drean A, Lemaire N, Bouchoux G, Goldwirt L, Canney M, Goli L, Bouzidi A, Schmitt C, Guehennec J, Verreault M, Sanson M, Delattre JY, Mokhtari K, Sottilini F, Carpentier A, Idbaih A (2019) Temporary blood-brain barrier disruption by low intensity pulsed ultrasound increases carboplatin delivery and efficacy in preclinical models of glioblastoma. J Neurooncol 144:33–41. https://doi.org/10.1007/s11060-019-03204-0
Leinenga G, Gotz J (2015) Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer's disease mouse model. Sci Transl Med 7:233–278. https://doi.org/10.1126/scitranslmed.aaa2512
Clement GT, White J, Hynynen K (2000) Investigation of a large-area phased array for focused ultrasound surgery through the skull. Phys Med Biol 45:1071–1083. https://doi.org/10.1088/0031-9155/45/4/319
Hynynen K, Jolesz FA (1998) Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol 24:275–283
McDannold N, Clement GT, Black P, Jolesz F, Hynynen K (2010) Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery 66:323–332. https://doi.org/10.1227/01.NEU.0000360379.95800.2F (discussion 332)
Crake C, Brinker ST, Coviello CM, Livingstone MS, McDannold NJ (2018) A dual-mode hemispherical sparse array for 3D passive acoustic mapping and skull localization within a clinical MRI guided focused ultrasound device. Phys Med Biol 63:065008. https://doi.org/10.1088/1361-6560/aab0aa
Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, Heyn C, Alkins R, Trudeau M, Sahgal A, Perry J, Hynynen K (2019) Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep 9:321. https://doi.org/10.1038/s41598-018-36340-0
Beccaria K, Canney M, Goldwirt L, Fernandez C, Adam C, Piquet J, Autret G, Clement O, Lafon C, Chapelon JY, Carpentier A (2013) Opening of the blood-brain barrier with an unfocused ultrasound device in rabbits. J Neurosurg 119:887–898. https://doi.org/10.3171/2013.5.JNS122374
Goldwirt L, Canney M, Horodyckid C, Poupon J, Mourah S, Vignot A, Chapelon JY, Carpentier A (2016) Enhanced brain distribution of carboplatin in a primate model after blood-brain barrier disruption using an implantable ultrasound device. Cancer Chemother Pharmacol 77:211–216. https://doi.org/10.1007/s00280-015-2930-5
Raymond SB, Treat LH, Dewey JD, McDannold NJ, Hynynen K, Bacskai BJ (2008) Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer's disease mouse models. PLoS ONE 3:e2175. https://doi.org/10.1371/journal.pone.0002175
Jordao JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, Hynynen K, Aubert I (2010) Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PLoS ONE 5:e10549. https://doi.org/10.1371/journal.pone.0010549
Jordao JF, Thevenot E, Markham-Coultes K, Scarcelli T, Weng YQ, Xhima K, O'Reilly M, Huang Y, McLaurin J, Hynynen K, Aubert I (2013) Amyloid-beta plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol 248:16–29. https://doi.org/10.1016/j.expneurol.2013.05.008
Kinoshita M, McDannold N, Jolesz FA, Hynynen K (2006) Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci USA 103:11719–11723. https://doi.org/10.1073/pnas.0604318103
Nisbet RM, Van der Jeugd A, Leinenga G, Evans HT, Janowicz PW, Gotz J (2017) Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain 140:1220–1230. https://doi.org/10.1093/brain/awx052
Janowicz PW, Leinenga G, Gotz J, Nisbet RM (2019) Ultrasound-mediated blood-brain barrier opening enhances delivery of therapeutically relevant formats of a tau-specific antibody. Sci Rep 9:9255. https://doi.org/10.1038/s41598-019-45577-2
Park EJ, Zhang YZ, Vykhodtseva N, McDannold N (2012) Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release 163:277–284. https://doi.org/10.1016/j.jconrel.2012.09.007
Kobus T, Zervantonakis IK, Zhang Y, McDannold NJ (2016) Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J Control Release 238:281–288. https://doi.org/10.1016/j.jconrel.2016.08.001
Alkins R, Burgess A, Kerbel R, Wels WS, Hynynen K (2016) Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro-oncology 18:974–981. https://doi.org/10.1093/neuonc/nov318
Chen PY, Hsieh HY, Huang CY, Lin CY, Wei KC, Liu HL (2015) Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J Transl Med 13:93. https://doi.org/10.1186/s12967-015-0451-y
Liu HL, Hsu PH, Lin CY, Huang CW, Chai WY, Chu PC, Huang CY, Chen PY, Yang LY, Kuo JS, Wei KC (2016) Focused ultrasound enhances central nervous system delivery of bevacizumab for malignant glioma treatment. Radiology 281:99–108. https://doi.org/10.1148/radiol.2016152444
Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK, Bresler M, Burks SR, Frank JA (2017) Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci USA 114:E75–E84. https://doi.org/10.1073/pnas.1614777114
Kovacs ZI, Burks SR, Frank JA (2018) Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: attention to experimental conditions. Theranostics 8:2245–2248. https://doi.org/10.7150/thno.24181
McMahon D, Hynynen K (2017) Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics 7:3989–4000. https://doi.org/10.7150/thno.21630
McMahon D, Hynynen K (2018) Reply to Kovacs et al.: concerning acute inflammatory response following focused ultrasound and microbubbles in the brain. Theranostics 8:2249–2250. https://doi.org/10.7150/thno.25468
Stavarache MA, Petersen N, Jurgens EM, Milstein ER, Rosenfeld ZB, Ballon DJ, Kaplitt MG (2018) Safe and stable noninvasive focal gene delivery to the mammalian brain following focused ultrasound. J Neurosurg 130:989–998. https://doi.org/10.3171/2017.8.JNS17790
McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K (2005) MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol 31:1527–1537. https://doi.org/10.1016/j.ultrasmedbio.2005.07.010
Liu HL, Wai YY, Hsu PH, Lyu LA, Wu JS, Shen CR, Chen JC, Yen TC, Wang JJ (2010) In vivo assessment of macrophage CNS infiltration during disruption of the blood-brain barrier with focused ultrasound: a magnetic resonance imaging study. J Cereb Blood Flow Metab 30:177–186. https://doi.org/10.1038/jcbfm.2009.179
Parsa AT, Chakrabarti I, Hurley PT, Chi JH, Hall JS, Kaiser MG, Bruce JN (2000) Limitations of the C6/Wistar rat intracerebral glioma model: implications for evaluating immunotherapy. Neurosurgery 47:993–999 (discussion 999–1000)
Zhu L, Cheng G, Ye D, Nazeri A, Yue Y, Liu W, Wang X, Dunn GP, Petti AA, Leuthardt EC, Chen H (2018) Focused ultrasound-enabled brain tumor liquid biopsy. Sci Rep 8:6553. https://doi.org/10.1038/s41598-018-24516-7
Acknowledgements
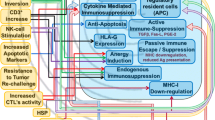
The authors acknowledge graphic designer Quentin Beccaria for his help in creating Fig. 1. Special thanks go to David M. Wildrick, Ph.D. and Audria Patrick for editorial and administrative support.
Author information
Authors and Affiliations
Contributions
Manuscript writing: KB, ABH. Manuscript editing: KB, MC, AC, JG, ABH, AS.
Corresponding author
Ethics declarations
Conflict of interest
M. Canney is an employee of CarThera. A. Carpentier is a paid consultant to CarThera. K. Beccaria was previously an employee of CarThera. A. Carpentier, K. Beccaria, and M. Canney are inventors on intellectual property related to the SonoCloud device that has been licensed to CarThera. A. Carpentier and M. Canney have ownership interested in CarThera.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beccaria, K., Sabbagh, A., de Groot, J. et al. Blood–brain barrier opening with low intensity pulsed ultrasound for immune modulation and immune therapeutic delivery to CNS tumors. J Neurooncol 151, 65–73 (2021). https://doi.org/10.1007/s11060-020-03425-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03425-8