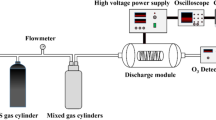
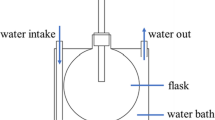
Abstract
A series Cu decorated MnO2/γ-Al2O3 catalysts (Cu load, 1%, 3% and 5%) was fabricated by facile co-impregnation method, and investigated for the catalytic oxidative removal Na2S in waste water. Compared with MnO2/γ-Al2O3, the catalytic activity and stability of Cu-MnO2/γ-Al2O3, was enhanced by modifying with Cu element. XRD, SEM, BET, H2-TPR and XPS were carried out to characterize these catalyst. XRD and BET show the Cu successfully decorated on the MnO2/γ-Al2O3, and catalyst’s pristine structure are well maintained. SEM shows that the doped Cu species benefits to the uniform dispersion of Mn species, H2-TPR suggests that the reodox capacity of Cu decorated catalysts were improved after doping with Cu, and XPS shows that doped Cu produces higher concentration of Mn4+ and Oads (surface active oxygen species), also expediting the redox cycling between Mn4+/Mn3+ and the promote the Oads production, which resulting in the enhancement of redox properties. Hence, 3%Cu-MnO2/γ-Al2O3 catalyst could achieve 97.5% degradation of Na2S in waste water at room conditions in 2 h, by the help of air, this result is superior to other published work.







Similar content being viewed by others
References
Vaiopoulou E, Melidis P, Aivasidis A (2005) Water Res 39:4101–4109
Altaş L, Büyükgüngör H (2008) J Hazard Mater 153:462–469
Rodriguez A, Ovejero G, Romero MD, Diaz C, Barreiro M (2008) J Supercrit Fluids 46:163–172
Hurse TJ, Abeydeera WPP (2002) J Chromatogr A 942:201–210
Nhi BD, Maratovich AR, Garipovna AA, Ivanova AS (2014) J Sulfur Chem 35:74–85
Vu ND, Bui ND, Minh TT, Dam HTT, Hang TT (2016) J Electron Mater 45:2316–2321
Hoffmann MR, Lim BC (1979) Environ Sci Technol 13:1406–1414
Allen SE, Walvoord RR, Rosaura PS, Kozlowski MC (2013) Chem Rev 113:6234–6458
Wenfeng WU, Zaihui FU, Tang S, Shuai Z, Wen XU, Yue M, Sun S, Jie D, Liu Y, Yin D (2015) Appl Catal B 164:113–119
Widger LR, Siegler MA, Goldberg DP (2013) Polyhedron 58:179–189
Speier G, Bagi N, Kaizer J (2015) Rsc Adv 5:45983–45986
Song G, Wang F, Zhang H, Lu X, Wang C (1998) Synth Commun 28:2783–2787
Ye W, Yang W, Yin X, Ying L (2016) J Environ Eng 4:3415–3425
Velusamy S, Kumar AV, Saini R, Punniyamurthy T (2005) Tetrahedron Lett 46:3819–3822
Dell'Anna MM, Mastrorilli P, Nobile CF (1996) J Mol Catal A 108:57–62
Liao X, Hu Z, Ma L, Hao Q, Lu S (2018) Chem Eng J 351:280–294
Liang Q, Xiaodong WU, Duan W, Haibo XU (2008) Catal Today 139:113–118
Pei J, Xu H, Yi L (2015) Build Environ 84:134–141
Elmhamdi A, Pascual L, Nahdi K, Martínez-Arias A (2017) Appl Catal B 217:1–11
Roshani B, Mcmaster I, Rezaei E, Soltan J (2014) Sep Purif Technol 135:158–164
Prabu K, Prabu M, Venugopal AK, Venugopalan AT, Sandilya WVYS, Gopinath CS, Raja T (2016) Appl Catal A 525:237–246
He S, Luan P, Mo L, Xu J, Li J, Zhu L, Zeng J (2018) BioResources 13:3686–3703
Yoo JB, Bang S, Kim K, Li C, Ji MK, Hur N (2016) Rsc Adv 6:113682–113688
Carvalho LMD, Schwedt G (2005) J Chromatogr A 1099:185–190
Zhang T, Wang D, Gao Z, Zhao K, Gu Y, Zhang Y, He D (2016) Rsc Adv 6:70261–70270
Jing L, Zhu P, Zuo S, Huang Q, Zhou R (2010) Appl Catal A 381:261–266
Cai L, Hu Z, Branton P, Li W (2014) Chin J Catal 35:159–167
Wagloehner S, Nitzer-Noski M, Kureti S (2015) Chem Eng J 259:492–504
Chen H, Sayari A, Adnot A, Larachi FÇ (2001) Appl Catal B 32:195–204
Liu Z, Hao J, Fu L, Zhu T, Li J, Cui X (2004) Appl Catal B 48:37–48
Yang Y, Huang J, Wang S, Deng S, Wang B, Gang Y (2013) Appl Catal B 142:568–578
Jia J, Zhang P, Long C (2016) Appl Catal B 189:210–218
Min W, Zhang L, Huang W, Zhou Y, Han Z, Jian L, Tian J, Kan X, Shi J (2017) Rsc Adv 7:14809–14815
Bai B, Li J, Hao J (2015) AAppl Catal B 164:241–250
Qi L, Yu Q, Dai Y, Tang C, Liu L, Zhang H, Gao F, Dong L, Chen Y (2012) Appl Catal B 119:308–320
Sun Y, Yang Z, Tian P, Sheng Y, Xu J, Han Y-F (2019) Appl Catal B 244:1–10
Xin Z, Tang Y, Qu S, Da J, Hao Z (2015) Acs Catal 5:1053–1067
Kantcheva M (2001) J Catal 204:479–494
Toops TJ, Smith DB, Epling WS, Parks JE, Partridge WP (2005) Appl Catal B 58:255–264
Zhu W, Wang C, Li H, Wu P, Xun S, Jiang W, Chen Z, Zhao Z, Li H (2015) Green Chem 17:2464–2472
Wang X, Yao J, Wang S, Pan X, Xiao R, Huang Q, Wang Z, Qu R (2018) Chemosphere 201:224–233
García-Gutiérrez JL, Fuentes GA, Hernández-Terán ME, Murrieta F, Navarrete J, Jiménez-Cruz F (2006) Appl Catal A 305:15–20
Hu L, Chen W, Xie X, Liu N, Yang Y, Wu H, Yao Y, Pasta M, Alshareef HN, Cui Y (2011) ACS Nano 5:8904–8913
Andersen NI, Serov A, Atanassov P (2015) Appl Catal B 163:623–627
Acknowledgements
This work was supported by the Project from the Tianjin Education Commission (2018KJ111).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cui, W., Zhao, R., Huang, X. et al. Cu doped MnO2/γ-Al2O3: a facile and efficient catalyst for the degradation of Na2S in waste water under ambient conditions. Reac Kinet Mech Cat 129, 1047–1059 (2020). https://doi.org/10.1007/s11144-020-01755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01755-2