Abstract—
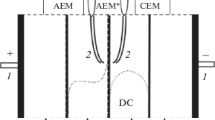
Electroconvection is the principal mechanism that allows markedly increasing the rate of ion transfer through ion-exchange membranes in intensive current regimes. In this work, we investigated the possibility of intensifying electroconvection in solution near heterogeneous MA-41 anion-exchange membrane (Shchekinoazot production) by the modifying of its surface. The use of weakly crosslinked ion-exchange resin (MA-41P) in the course of the membrane manufacturing, with subsequent chemical modification of its surface (MA-41PM), is shown to make it possible to increase the limiting current density almost twice. The value of the reduced potential drop (after subtracting the ohmic contribution), at which significant generation of H+ and OH– ions begins, is shifted from 0.8 V in the case of MA-41 to 1.7 V in the case of MA-41PM. The current density related to the onset of water splitting is equal to 0.9\(i_{{{\text{lim}}}}^{{{\text{Lev}}}},\) in the case of MA-41; 2\(i_{{{\text{lim}}}}^{{{\text{Lev}}}},\) in the case of MA-41PM (where \(i_{{{\text{lim}}}}^{{{\text{Lev}}}}\) is the theoretical value of the limiting current density). The special feature of the modified membrane behavior is the presence of a range of potential drop (between 50 and 80 mV in the reduced scale), in which the system with the MA-41PM has negative differential resistance: in this range, the potential drop decreases when the current density increases. This behavior occurs when measuring quasi-stationary I–V curves; correspondingly, in the chronopotentiogram there is a time interval, where the potential drop decreases with time. The electroconvection is intensified near a modified membrane due to a higher fraction of conductive areas on the surface of the modified membrane and the redistribution of these areas via formation of their agglomerates in the centers of the cells formed by the reinforcing mesh. Mathematical modeling shows the concentration polarization of the modified membrane being less than that of the pristine one. Meanwhile, the structure of electroconvective vortices is optimized: the vortices near the modified membrane are larger; they do not extinguish each other, unlike the case of MA-41.









Similar content being viewed by others
REFERENCES
Strathmann, H., Grabowski, A., and Eigenberger, G., Ion-Exchange Membranes in the Chemical Process Industry, Ind. Eng. Chem. Res., 2013, vol. 52, no. 31, p. 10364.
Zaltzman, B. and Rubinstein, I., Electro-osmotic slip and electroconvective instability, J. Fluid Mech., 2007, vol. 579, p. 173.
Mishchuk, N.A. Concentration polarization of interface and non-linear electrokinetic phenomena, Adv. Colloid Interface Sci., 2010, vol. 160, nos. 1–2, p. 16.
Tanaka, Y., Ion Exchange Membranes, Fundamentals and Applications, vol. 12 2nd Edition, Elsevier Science, 2015.
Vasil’eva, V.I., Akberova, E.M., and Zabolotskii, V.I., Electroconvection in systems with heterogeneous ion-exchange membranes after thermal modification, Russ. J. Electrochem., 2017, vol. 53, no. 4, p. 398.
Nikonenko, V.V., Mareev, S.A., Pis’menskaya, N.D., Uzdenova, A.M., Kovalenko, A.V., Urtenov, M.Kh., and Pourcelly, G., Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis (Review), Russ. J. Electrochem., 2017, vol. 53, no. 10, p. 1122.
Pawlowski, S., Crespo, J., Velizarov, S., Crespo J., and Velizarov, S., Profiled Ion Exchange Membranes: A Comprehensible Review, Int. J. Mol. Sci., 2019, vol. 20, no 1, p. 165.
Belloň, T., Polezhaev, P., Vobecká, L., Svoboda, M., and Slouka, Z. Experimental observation of phenomena developing on ion-exchange systems during current-voltage curve measurement, J. Memb. Sci., 2019, vol. 572, p. 607.
Belova, E., Lopatkova, G., Pismenskaya, N., Nikonenko, V., Larchet, C., and Pourcelly, G., Effect of anion-exchange membrane surface properties on mechanisms of overlimiting mass transfer, J. Phys. Chem. B, 2006, vol. 110, no. 27, p. 13458.
Sharafan, M., Zabolotskii, V., and Bugakov, V., Electric mass transport through homogeneous and surface-modified heterogeneous ion-exchange membranes at a rotating membrane disk, Russ. J. Electrochem., 2009, vol. 45, no. 10, p. 1162.
Mikhaylin, S., and Bazinet, L., Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control, Adv. Colloid Interface Sci., 2016, vol. 229, p. 34.
Mikhaylin, S., Nikonenko, V., Pismenskaya, N., Pourcelly, G., Choi, S., Kwon, H., Han, J., H., and Bazinet, L., How physico-chemical and surface properties of cation-exchange membrane affect membrane scaling and electroconvective vortices: Influence on performance of electrodialysis with pulsed electric field, Desalination., 2016, vol. 393, p. 102.
Andreeva, M., Gil, V., Pismenskaya, N., Dammak, L., Kononenko, N., Larchet, C., Grande, D., and Nikonenko, V., Mitigation of membrane scaling in electrodialysis by electroconvection enhancement, pH adjustment and pulsed electric field application, J. Memb. Sci., 2018, vol. 549, p. 129.
Lemay, N., Mikhaylin, S., and Bazinet, L., Voltage spike and electroconvective vortices generation during electrodialysis under pulsed electric field: Impact on demineralization process efficiency and energy consumption, Innov. Food Sci. Emerg. Technol., 2019, vol. 52, p. 221.
Bazant, M., Kilic, M., Storey, B., and Ajdari, A., Towards an understanding of induced-charge electrokinetics at large applied voltages in concentrated solutions, Adv. Colloid Interface Sci., 2009, vol. 152, nos. 1–2, p. 48.
Olesen, L., Bruus H., and Ajdari, A., AC electrokinetic micropumps: The effect of geometrical confinement, Faradaic current injection, and nonlinear surface capacitance, Phys. Rev. E, 2006, vol. 73, no. 5, p. 056313.
Zhou, C., Zhang, H., Li, Z., and Wang, W., Chemistry pumps: a review of chemically powered micropumps, Lab on a Chip, 2016, vol. 16, no. 10, p. 1797.
Wang, Y., Stevens A., and Han, J., Million-fold Preconcentration of Proteins and Peptides by Nanofluidic Filter, Analyt. Chem., 2005, vol. 77, no. 14, p. 4293.
Mani, A. and Bazant, M., Deionization shocks in microstructures, Phys. Rev. E – Stat. Nonlinear, and Soft Matter Phys., 2011, vol. 84, p. 1.
Yaroshchuk, A., Over-limiting currents and deionization shocks in current-induced polarization: Local-equilibrium analysis, Adv. Colloid Interface Sci., 2012, vols. 183–184, p. 68.
Sia, S. and Whitesides, G., Microfluidic devices fabricated in Poly(dimethylsiloxane) for biological studies, Electrophoresis, 2003, vol. 24, no. 21, p. 3563.
Sackmann, E., Fulton, A., and Beebe, D., The present and future role of microfluidics in biomedical research, Nature, 2014, vol. 507, no. 7491, p. 181.
de Jong, J., Lammertink, R., and Wessling, M., Membranes and microfluidics: a review, Lab on a Chip, 2006, vol. 6, no. 9, p. 1125.
Slouka, Z., Senapati, S., and Chang, H., Microfluidic Systems with Ion-Selective Membranes, Annu. Rev. Anal. Chem, 2014, vol. 7, no. 1, p. 317.
Akberova, E., Vasil’eva, V., Zabolotsky, V., and Novak, L., Effect of the sulfocation-exchanger dispersity on the surface morphology, microrelief of heterogeneous membranes and development of electroconvection in intense current modes, J. Memb. Sci., 2018, vol. 566, p. 317.
Pismenskaya, N., Pokhidnia, E., Pourcelly, G., and Nikonenko, V., Can the electrochemical performance of heterogeneous ion-exchange membranes be better than that of homogeneous membranes?, J. Memb. Sci. 2018, vol. 566, p. 54.
Zabolotsky, V., Novak, L., Kovalenko, A., Nikonenko, V., Urtenov, M., Lebedev, K., and But, A., Electroconvection in systems with heterogeneous ion-exchange membranes, Pet. Chem., 2017, vol. 57, no. 9, p. 779.
Pismenskaya, N., Belova, E., Nikonenko, V., Zabolotsky, V., Lopatkova, G., Karzhavin, Yu., and Larchet, C., Lower rate of H+ (OH−) ions generation at an anion-exchange membrane in electrodialysis, Desalin. Water Treat., 2010, vol. 21, nos. 1–3, p. 109.
Zabolotskiy, V., But, A., Vasil’eva, V., Akberova, E., Melnikov, S., Vasil, V., Akberova, E., and Melnikov, S., Ion transport and electrochemical stability of strongly basic anion-exchange membranes under high current electrodialysis conditions, J. Memb. Sci., 2017, vol. 526, p. 60.
Rubinstein, I., Zaltzman, B., and Pundik, T., Ion-exchange funneling in thin-film coating modification of heterogeneous electrodialysis membranes, Phys. Rev. E – Stat. Nonlinear, Soft Matter Phys., 2002, vol. 65, no. 4, p. 1.
Davidson, S.M., Wessling, M., and Mani, A., On the Dynamical Regimes of Pattern-Accelerated Electroconvection, Sci. Rep., 2016, vol. 6, no. 1, p. 22505.
Mareev, S., Nichka, V., Butylskii, D., Urtenov, M., Pismenskaya, N., Apel, P., and Nikonenko, V., Chronopotentiometric Response of an Electrically Heterogeneous Permselective Surface: 3D Modeling of Transition Time and Experiment, J. Phys. Chem. C, 2016, vol. 120, no. 24, p. 13113.
Mareev, S., Nebavskiy, A., Nichka, V., Urtenov, M., and Nikonenko, V., The nature of two transition times on chronopotentiograms of heterogeneous ion exchange membranes: 2D modelling, J. Memb. Sci., 2019, vol. 575, p. 179.
Slavinskaya, G.V. and Kurenkova, O.V., On the multifunctional character of strong basic anion-exchange resin, Sorption and Chromatographic Processes (in Russian), 2019, vol. 19, no. 1, p. 101.
Belashova, E., Melnik, N., Pismenskaya, N., Shevtsova, K., Nebavsky, A., Lebedev, K., and Nikonenko, V., Overlimiting mass transfer through cation-exchange membranes modified by Nafion film and carbon nanotubes, Electrochim. Acta, 2012, vol. 59, p. 412.
Helfferich, F., Ion Exchange, New York: McGraw-Hill, 1962.
Lteif, R., Dammak, L., Larchet, C., and Auclair, B., Conductivitéélectrique membranaire: étude de l’effet de la concentration, de la nature de l’électrolyte et de la structure membranaire, Eur. Polym. J., 1999, vol. 35, no. 7, p. 1187.
Nikonenko, V.V., Vedernikova E.E., and Pismen-skaya, N.D., Patent 100275 (Russia), 2010.
Larchet, C., Auclair, B., and Nikonenko, V., Approximate evaluation of water transport number in ion-exchange membranes, Electrochim. Acta, 2004, vol. 49, no. 11, p. 1711.
Zabolotsky, V. and Nikonenko, V., Effect of structural membrane inhomogeneity on transport properties, J. Memb. Sci., 1993, vol. 79, p. 181.
Pismenskaya, N., Nikonenko, V., Melnik, N., Shevtsova, K., Belova, E., Pourcelly, G, Cot, D, Dammak, L, and Larchet, C., Evolution with time of hydrophobicity and microrelief of a cation-exchange membrane surface and its impact on overlimiting mass transfer, J. Phys. Chem. B, 2012, vol. 116, no. 7, p. 2145.
Newman, J.S. and Thomas-Alyea K.E. Electrochemical systems.New Jersey:John Wiley & Sons, 2004, p. 647.
Peers, A.M., Membrane phenomena, Discuss. Faraday Soc., 1956, vol. 21, p. 124.
Gnusin, N.P., Zabolotskii, V.I., Urtenov, M.H., and Nikonenko, V.V., Convective-diffusion model of electrodialytic desalination. limiting current and diffusion layer.,Sov. Electrochem., 1986, vol. 23, no. 3, p. 273.
Rubinstein, I. and Zaltzman, B., Electro-osmotically induced convection at a permselective membrane, Phys. Rev. E., 2000, vol. 62, no. 2, p. 2238.
Nikonenko, V., Pismenskaya, N., Belova, E., Sistat, P., Huguet, P., Pourcelly, G., and Larchet, C., Intensive current transfer in membrane systems: Modelling, mechanisms and application in electrodialysis, Adv. Colloid Interface Sci., 2010, vol. 160, nos. 1–2, p. 101.
Kharkats, Yu.I., Mechanism of ‘supralimiting’ currents at ion-exchange membrane/electrolyte interfaces., Sov. Electrochem., 1985, vol. 21, p. 917.
Block, M. and Kitchener, J.A., Polarization Phenomena in Commercial Ion-Exchange Membranes, J. Electrochem. Soc., 1966, vol. 113, no. 9, p. 947.
Van Soestbergen, M., Biesheuvel, P.M., and Bazant, M.Z., Diffuse-charge effects on the transient response of electrochemical cells, Phys. Rev. E – Stat. Nonlinear, Soft Matter Phys., 2010, vol. 81, no. 2, p. 1.
Mareev, S., Butylskii, D., Pismenskaya, N., and Nikonenko, V., Chronopotentiometry of ion-exchange membranes in the overlimiting current range. Transition time for a finite-length diffusion layer: modeling and experiment, J. Memb. Sci., 2016, vol. 500, p. 171.
Volodina, E., Pismenskaya, N., Nikonenko, V., Larchet, C., and Pourcelly, G., Ion transfer across ion-exchange membranes with homogeneous and heterogeneous surfaces, J. Colloid Interface Sci., 2005, vol. 285, no. 1, p. 247.
Martí-Calatayud, M.C., Buzzi, D.C., García-Gabaldón, M., Bernardes, A.M., Tenório, J.A., and Pérez-Herranz, V., Ion transport through homogeneous and heterogeneous ion-exchange membranes in single salt and multicomponent electrolyte solutions, J. Memb. Sci., 2014, vol. 466, p. 45.
Butylskii, D., Mareev, S., Pismenskaya, N., Apel, P., Polezhaeva, O., and Nikonenko, V., Phenomenon of two transition times in chronopotentiometry of electrically inhomogeneous ion exchange membranes, Electrochim. Acta, 2018, vol. 273, p. 289.
Rubinstein, I. and Zaltzman, B. Equilibrium Electroconvective Instability, Phys. Rev. Lett., 2015, vol. 114, no. 11, p. 114502.
Abu-Rjal, R., Prigozhin, L., Rubinstein, I., and Zaltzman, B., Equilibrium electro-convective instability in concentration polarization: The effect of non-equal ionic diffusivities and longitudinal flow, Russ. J. Electrochem., 2017, vol. 53, p. 903.
Mishchuk, N.A., Electro-osmosis of the second kind near the heterogeneous ion-exchange membrane, Colloids Surfaces A Physicochem. Eng. Asp., 1998, vol. 140, nos. 1–3, p. 75.
Mareev, S.A., Butylskii, D., Pismenskaya, N., Larchet, C., Dammak, L., and Nikonenko, V., Geometric heterogeneity of homogeneous ion-exchange Neosepta membranes, J. Memb. Sci., 2018, vol. 563, p. 768.
Korzhova, E., Pismenskaya, N., Lopatin, D., Baranov, O., Dammak, L., and Nikonenko, V., Effect of surface hydrophobization on chronopotentiometric behavior of an AMX anion-exchange membrane at overlimiting currents, J. Memb. Sci., 2016, vol. 500, p. 161.
Nebavskaya, K., Sarapulova, V., Sabbatovskiy, K., Sobolev, V., Pismenskaya, N., Sistat, P., Cretin, M., and Nikonenko, V., Impact of ion exchange membrane surface charge and hydrophobicity on electroconvection at underlimiting and overlimiting currents, J. Memb. Sci., 2017, vol. 523, p. 36.
Belova E., Lopatkova, G., Pismenskaya, N., Nikonenko, V., and Larchet, C., Role of water splitting in development of electroconvection in ion-exchange membrane systems, Desalination., 2006, vol. 199, nos. 1–3, p. 59.
Slouka Z., Senapati, S., Yan, Yu., and Chang, H.-C.C., Charge inversion, water splitting, and vortex suppression due to DNA sorption on ion-selective membranes and their ion-current signatures, Langmuir, 2013, vol. 29, no. 26, p. 8275.
Vasil’eva, V., Zhil’tsova, A., Malykhin, M., Zabolotskii, V., Lebedev, K., Chermit, R., and Sharafan, M., Effect of the chemical nature of the ionogenic groups of ion-exchange membranes on the size of the electroconvective instability region in high-current modes, Russ. J. Electrochem., 2014, vol. 50, p. 120.
Andreeva, M., Gil, V., Pismenskaya, N., Nikonenko, V., Dammak, L., Larchet, C., Grande, D., and Kononenko, N., Effect of homogenization and hydrophobization of a cation-exchange membrane surface on its scaling in the presence of calcium and magnesium chlorides during electrodialysis, J. Memb. Sci., 2017, vol. 540, p. 183.
Dukhin, S.S., Electrokinetic phenomena of the second kind and their applications, Adv. Colloid Interface Sci., 1991, vol. 35, p. 173.
Urtenov, M., Uzdenova, A., Kovalenko, A., Nikonenko, V., Pismenskaya, N., Vasil’eva, V., Sistat, P., and Pourcelly, G., Basic mathematical model of overlimiting transfer enhanced by electroconvection in flow-through electrodialysis membrane cells, J. Memb. Sci., 2013, vol. 447, p. 190.
Ganchenko, G., Kalaydin, E., Schiffbauer, J., and Demekhin, E., Modes of electrokinetic instability for imperfect electric membranes, Phys. Rev. E., 2016, vol. 94, no. 6, p. 063106.
Rubinstein, I. and Maletzki, F., Electroconvection at an electrically inhomogeneous permselective membrane surface, J. Chem. Soc. Faraday Trans., 1991, vol. 87, no. 13, p. 2079.
Pham, V.S., Li, Z., Lim, K.M., White, J.K., and Han, J., Direct numerical simulation of electroconvective instability and hysteretic current-voltage response of a permselective membrane, Phys. Rev. E - Stat. Nonlinear, Soft Matter Phys., 2012, vol. 86, p. 1.
Nikonenko, V. V., Yaroslavtsev, A.B., and Pourcelly, G., Ion Transfer in and Through Charged Membranes: Structure, Properties, and Theory, in Ionic Interactions in Natural and Synthetic Macromolecules, 2012. Chapter 9, p. 267.
ACKNOWLEDGEMENTS
Authors are grateful to the core facilities center “Ecology and analytical center” of Kuban State University and Laboratory “Metals and Ceramics with Controlled Microstructures,” Metallurgy and Inorganic Chemistry Department at the East Paris Institute of Chemistry and Materials Science, CNRS, France.
Funding
The work is carried out in the Russian–French International associated laboratory “ion-exchange membranes and processes” under the sponsorship of Ministry of Science and Higher Education of the Russian Federation (Ref. no. RFMEFI58617X0030) and CNRS, France (project no. 38200SF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Yu. Pleskov
APPENDIX
APPENDIX
Let us examine thin solution layer with thickness of ∆x\( \ll \) δ adjacent to a membrane surface. We write the counter-ion flux (for definiteness, of single-charged cations in the case of cation-exchange membrane) entering this layer from the solution bulk as
where \(D{{\left( {c_{1}^{0} - {{c}_{{{\text{1s}}}}}} \right)} \mathord{\left/ {\vphantom {{\left( {c_{1}^{0} - {{c}_{{{\text{1s}}}}}} \right)} \delta }} \right. \kern-0em} \delta }\) is the finite-difference estimate of the flow diffusion component, \(c_{1}^{0}\) and \({{c}_{{1\,{\text{s}}}}}\) is the counter-ion concentration in the solution bulk and near the membrane surface, respectively. We write the flux outward from this layer to the membrane bulk by using the definition of the effective transport number for ions 1 in the membrane, T1, as the portion of charge carried by the ions of this kind under the conditions of possible involvement of the migration and diffusion transfer mechanisms:
Because the contribution of the diffusion transfer in highly selective ion-exchange membranes in dilute solutions is insignificant, the value of T1 approaches the electromigration transport number \({{\bar {t}}_{1}}\) [69].
We write the differential equation of the ion 1 conservation in the layer under examination (per unit membrane surface area, mol m−2) as:
The rate of increment \({{\partial ({{c}_{{1\,{\text{s}}}}}\Delta x)} \mathord{\left/ {\vphantom {{\partial ({{c}_{{1\,{\text{s}}}}}\Delta x)} {\partial t}}} \right. \kern-0em} {\partial t}}\) gain depends on the current density i and time t. While in the initial state we have \({{c}_{{1\,{\text{s}}}}}\) = \(c_{1}^{0}\) (the system is at equilibrium) and there is no ion diffusion delivery to the membrane surface, upon the current switching-off the quantity \({{\partial ({{c}_{{1\,{\text{s}}}}}\Delta x)} \mathord{\left/ {\vphantom {{\partial ({{c}_{{1\,{\text{s}}}}}\Delta x)} {\partial t}}} \right. \kern-0em} {\partial t}}\) becomes negative (because T1 > t1) and has its maximal absolute value in time. As time goes by, \(\partial {{\left( {{{c}_{{1\,{\text{s}}}}}\Delta x} \right)} \mathord{\left/ {\vphantom {{\left( {{{c}_{{1\,{\text{s}}}}}\Delta x} \right)} {\partial t}}} \right. \kern-0em} {\partial t}}\) decreases in absolute value because \({{c}_{{1\,{\text{s}}}}}\) decreases, while \({{D(c_{1}^{0} - {{c}_{{1\,{\text{s}}}}})} \mathord{\left/ {\vphantom {{D(c_{1}^{0} - {{c}_{{1\,{\text{s}}}}})} \delta }} \right. \kern-0em} \delta }\) increases. When the current density remains not too high (less than its limiting value, ilim), the diffusion delivery can compensate the difference of migration fluxes in the solution and membrane, and the system reaches its steady state when \({{\partial ({{c}_{{1\,{\text{s}}}}}\Delta x)} \mathord{\left/ {\vphantom {{\partial ({{c}_{{1\,{\text{s}}}}}\Delta x)} {\partial t}}} \right. \kern-0em} {\partial t}}\) = 0. When i > ilim, then, upon the reaching of some sufficiently small threshold value of  , an additional mechanism of the current flowing becomes developing in the system (a current-induced convection or the generation of Н+ and ОН− ions, the new charge carriers). The appearance of the new transfer mechanism causes a decrease in the intramembrane potential drop growth rate and formation of inflection in the chronopotentiogram. The same mechanism (along with the diffusion from solution bulk) provides compensation of the difference in the ion migration fluxes and the achieving of the steady state.
, an additional mechanism of the current flowing becomes developing in the system (a current-induced convection or the generation of Н+ and ОН− ions, the new charge carriers). The appearance of the new transfer mechanism causes a decrease in the intramembrane potential drop growth rate and formation of inflection in the chronopotentiogram. The same mechanism (along with the diffusion from solution bulk) provides compensation of the difference in the ion migration fluxes and the achieving of the steady state.
It is not too difficult to see from equation (А3) that the less the diffusion layer thickness, the more intensive is the electrolyte diffusion delivery to the membrane surface, all other things being equal. This means that with the decreasing of δ the absolute value of \({{\partial ({{c}_{{1\,{\text{s}}}}}\Delta x)} \mathord{\left/ {\vphantom {{\partial ({{c}_{{1\,{\text{s}}}}}\Delta x)} {\partial t}}} \right. \kern-0em} {\partial t}}\) will decrease (remaining negative); a longer time will be required for the achieving of the critically low concentration near the surface.
Rights and permissions
About this article
Cite this article
Pismenskaya, N.D., Mareev, S.A., Pokhidnya, E.V. et al. Effect of Surface Modification of Heterogeneous Anion-Exchange Membranes on the Intensity of Electroconvection at Their Surfaces. Russ J Electrochem 55, 1203–1220 (2019). https://doi.org/10.1134/S1023193519120139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193519120139