Abstract
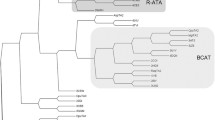
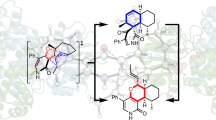
Pyridoxal-5′-phosphate-dependent transaminases of fold type IV (class IV) are promising enzymes for (R)-selective amination of organic compounds. Transaminases of fold type IV exhibit either strict (R)-selectivity or (S)-selectivity that is implemented within geometrically similar active sites of different amino acid compositions. Based on substrate specificity, class IV comprises three large families of transaminases: (S)-selective branched-chain L-amino acid aminotransferases and (R)-selective D-amino acid aminotransferases and (R)-amine:pyruvate transaminases. In this review, we aim to analyze the substrate profiles and correlations between the substrate specificity and organization of the active site in transaminases from these structurally related families. New transaminases with an expanded substrate specificity are also discussed. An analysis of the structural features of substrate binding and comparisons of structural determinants of chiral discrimination between members of the class IV transaminases could be helpful in identifying new biocatalytically relevant enzymes as well as rational protein engineering.







Similar content being viewed by others
References
Amorim Franco TM, Hegde S, Blanchard JS (2016) Chemical mechanism of the branched-chain aminotransferase IlvE from Mycobacterium tuberculosis. Biochemistry 55:6295–6303. https://doi.org/10.1021/acs.biochem.6b00928
Barber JEB, Damry AM, Calderini GF, Walton CJW, Chica RA (2014) Continuous colorimetric screening assay for detection of d-amino acid aminotransferase mutants displaying altered substrate specificity. Anal Biochem 463:23–30. https://doi.org/10.1016/j.ab.2014.06.006
Bezsudnova EY, Boyko KM, Nikolaeva AY, Zeifman YS, Rakitina TV, Suplatov DA, Popov VO (2019) Biochemical and structural insights into PLP fold type IV transaminase from Thermobaculum terrenum. Biochimie 158:130–138. https://doi.org/10.1016/j.biochi.2018.12.017
Bezsudnova EY, Boyko KM, Popov VO (2017) Properties of bacterial and archaeal branched-chain amino acid aminotransferases. Biochemistry (Mosc) 82:1572–1591. https://doi.org/10.1134/S0006297917130028
Bezsudnova EY, Dibrova DV, Nikolaeva AY, Rakitina TV, Popov VO (2018) Identification of branched-chain amino acid aminotransferases active towards (R)-(+)-1-phenylethylamine among PLP fold type IV transaminases. J Biotechnol 271:26–28. https://doi.org/10.1016/j.jbiotec.2018.02.005
Bezsudnova EY, Stekhanova TN, Suplatov DA, Mardanov AV, Ravin NV, Popov VO (2016) Experimental and computational studies on the unusual substrate specificity of branched-chain amino acid aminotransferase from Thermoproteus uzoniensis. Arch Biochem Biophys 607:27–36. https://doi.org/10.1016/j.abb.2016.08.009
Bhatia MB, Del Pozo AM, Ringe D, Yoshimura T, Soda K, Manning JM (1993) Role reversal for substrates and inhibitors. Slow inactivation of D-amino acid transaminase by its normal substrates and protection by inhibitors. J Biol Chem 268:17687–17694
Bornscheuer UT (2018) The fourth wave of biocatalysis is approaching. Philos Trans R Soc A Math Phys Eng Sci 376:20170063. https://doi.org/10.1098/rsta.2017.0063
Bornscheuer UT, Lutz S, Robins K, Kazlauskas RJ, Huisman GW, Moore JC (2012) Engineering the third wave of biocatalysis. Nature 485:185–194. https://doi.org/10.1038/nature11117
Boyko KM, Stekhanova TN, Nikolaeva AY, Mardanov AV, Rakitin AL, Ravin NV, Bezsudnova EY, Popov VO (2016) First structure of archaeal branched-chain amino acid aminotransferase from Thermoproteus uzoniensis specific for L-amino acids and R-amines. Extremophiles 20:215–225. https://doi.org/10.1007/s00792-016-0816-z
Brundiek H, Höhne M (2016) Transaminases - a biosynthetic route for chiral amines. In: Applied biocatalysis: from fundamental science to industrial applications. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 199–218
Busto E, Simon RC, Grischek B, Gotor-Fernández V, Kroutil W (2014) Cutting short the asymmetric synthesis of the ramatroban precursor by employing ω-transaminases. Adv Synth Catal 356:1937–1942. https://doi.org/10.1002/adsc.201300993
Chen C-D, Lin C-H, Chuankhayan P, Huang Y-C, Hsieh Y-C, Huang T-F, Guan H-H, Liu M-Y, Chang W-C, Chen C-J (2012) Crystal structures of complexes of the branched-chain aminotransferase from Deinococcus radiodurans with α-ketoisocaproate and L-glutamate suggest the radiation resistance of this enzyme for catalysis. J Bacteriol 194:6206–6216. https://doi.org/10.1128/JB.01659-12
Cooper AJL, Meister A (1989) An appreciation of professor Alexander E. Braunstein The discovery and scope of enzymatic transamination. Biochimie 71:387–404. https://doi.org/10.1016/0300-9084(89)90169-7
D’Este M, Alvarado-Morales M, Angelidaki I (2018) Amino acids production focusing on fermentation technologies – a review. Biotechnol Adv 36:14–25. https://doi.org/10.1016/j.biotechadv.2017.09.001
Dai YN, Chi CB, Zhou K, Cheng W, Jiang YL, Ren YM, Ruan K, Chen Y, Zhou CZ (2013) Structure and catalytic mechanism of yeast 4-amino-4-deoxychorismate lyase. J Biol Chem 288:22985–22992. https://doi.org/10.1074/jbc.M113.480335
Desai AA (2011) Sitagliptin manufacture: a compelling tale of Green chemistry, process intensification, and industrial asymmetric catalysis. Angew Chemie Int Ed 50:1974–1976. https://doi.org/10.1002/anie.201007051
Dold S-M, Syldatk C, Rudat J (2016) Transaminases and their applications. In: Green biocatalysis. John Wiley & Sons, Inc, Hoboken, NJ, pp 715–746
Dunathan HC (1966) Conformation and reaction specificity in pyridoxal phosphate enzymes. Proc Natl Acad Sci U S A 55:712–716
Dunbabin A, Subrizi F, Ward JM, Sheppard TD, Hailes HC (2017) Furfurylamines from biomass: transaminase catalysed upgrading of furfurals. Green Chem 19:397–404. https://doi.org/10.1039/C6GC02241C
Eliot AC, Kirsch JF (2004) Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations. Annu Rev Biochem 73:383–415. https://doi.org/10.1146/annurev.biochem.73.011303.074021
Ferrandi EE, Monti D (2018) Amine transaminases in chiral amines synthesis: recent advances and challenges. World J Microbiol Biotechnol 34:13–10. https://doi.org/10.1007/s11274-017-2395-2
Fesko K, Steiner K, Breinbauer R, Schwab H, Schürmann M, Strohmeier GA (2013) Investigation of one-enzyme systems in the ω-transaminase-catalyzed synthesis of chiral amines. J Mol Catal B Enzym 96:103–110. https://doi.org/10.1016/j.molcatb.2013.06.015
Fuchikami Y, Yoshimura T, Gutierrez A, Soda K, Esaki N (1998) Construction and properties of a fragmentary D-amino acid aminotransferase. J Biochem 124:905–910. https://doi.org/10.1093/oxfordjournals.jbchem.a022206
Fuchs M, Farnberger JE, Kroutil W (2015) The industrial age of biocatalytic transamination. European J Org Chem 2015:6965–6982. https://doi.org/10.1002/ejoc.201500852
Gao X, Ma Q, Zhu H (2015) Distribution, industrial applications, and enzymatic synthesis of d-amino acids. Appl Microbiol and Biotechnol 99:3341-3349. https://doi.org/10.1007/s00253-015-6507-3
Gao S, Su Y, Zhao L, Li G, Zheng G (2017) Characterization of a (R)-selective amine transaminase from Fusarium oxysporum. Process Biochem 63:130–136. https://doi.org/10.1016/j.procbio.2017.08.012
Goldberg JM, Swanson RV, Goodman HS, Kirsch JF (1991) The tyrosine-225 to phenylalanine mutation of Escherichia coli aspartate aminotransferase results in an alkaline transition in the spectrophotometric and kinetic pKa values and reduced values of both kcat and km. Biochemistry 30:305–312. https://doi.org/10.1021/bi00215a041
Gomm A, Lewis W, Green AP, O’Reilly E (2016) A new generation of smart amine donors for transaminase-mediated biotransformations. Chem - A Eur J 22:12692–12695. https://doi.org/10.1002/chem.201603188
Goto M, Miyahara I, Hayashi H, Kagamiyama H, Hirotsu K (2003) Crystal structures of branched-chain amino acid aminotransferase complexed with glutamate and glutarate: true reaction intermediate and double substrate recognition of the enzyme. Biochemistry 42:3725–3733. https://doi.org/10.1021/bi026722f
Grishin DV, Zhdanov DD, Pokrovskaya MV, Sokolov NN (2020) D-amino acids in nature, agriculture and biomedicine. Front Life Sci 13:11–22. https://doi.org/10.1080/21553769.2019.1622596
Grishin NV, Phillips MA, Goldsmith EJ (1995) Modeling of the spatial structure of eukaryotic ornithine decarboxylases. Protein Sci 4:1291–1304. https://doi.org/10.1002/pro.5560040705
Griswold WR, Toney MD (2011) Role of the pyridine nitrogen in pyridoxal 5′-phosphate catalysis: activity of three classes of PLP enzymes reconstituted with deazapyridoxal 5′-phosphate. J Am Chem Soc 133:14823–14830. https://doi.org/10.1021/ja2061006
Guan L-J, Ohtsuka J, Okai M, Miyakawa T, Mase T, Zhi Y, Hou F, Ito N, Iwasaki A, Yasohara Y, Tanokura M (2015) A new target region for changing the substrate specificity of amine transaminases. Sci Rep 5:1–8. https://doi.org/10.1038/srep10753
Guo F, Berglund P (2017) Transaminase biocatalysis: optimization and application. Green Chem 19:333–360. https://doi.org/10.1039/C6GC02328B
Hayashi H (1995) Pyridoxal enzymes: mechanistic diversity and uniformity. J Biochem 118:463–473. https://doi.org/10.1093/oxfordjournals.jbchem.a124931
Hirotsu K, Goto M, Okamoto A, Miyahara I (2005) Dual substrate recognition of aminotransferases. Chem Rec 5:160–172. https://doi.org/10.1002/tcr.20042
Höhne M, Bornscheuer UT (2009) Biocatalytic routes to optically active amines. ChemCatChem 1:42–51. https://doi.org/10.1002/cctc.200900110
Höhne M, Schätzle S, Jochens H, Robins K, Bornscheuer UT (2010) Rational assignment of key motifs for function guides in silico enzyme identification. Nat Chem Biol 6:807–813. https://doi.org/10.1038/nchembio.447
Hult K, Berglund P (2007) Enzyme promiscuity: mechanism and applications. Trends Biotechnol 25:231–238. https://doi.org/10.1016/j.tibtech.2007.03.002
Hutson S (2001) Structure and function of branched chain aminotransferases. Prog Nucleic Acid Res Mol Biol 70:175–206. https://doi.org/10.1016/s0079-6603(01)70017-7
Iglesias C, Panizza P, Rodriguez Giordano S (2017) Identification, expression and characterization of an R-ω-transaminase from Capronia semiimmersa. Appl Microbiol Biotechnol 101:5677–5687. https://doi.org/10.1007/s00253-017-8309-2
Inoue K, Kuramitsu S, Aki K, Watanabe Y, Takagi T, Nishigai M, Ikai A, Kagamiyama H (1988) Branched-chain amino acid aminotransferase of Escherichia coli: overproduction and Properties1. J Biochem 104:777–784. https://doi.org/10.1093/oxfordjournals.jbchem.a122549
Inoue K, Kuramitsu S, Okamoto A, Hirotsu K, Higuchi T, Morino Y, Kagamiyama H (1991) Tyr225 in aspartate aminotransferase: contribution of the hydrogen bond between Tyr225 and coenzyme to the catalytic Reaction1. J Biochem 109:570–576. https://doi.org/10.1093/oxfordjournals.jbchem.a123421
Isupov MN, Boyko KM, Sutter J-M, James P, Sayer C, Schmidt M, Schönheit P, Nikolaeva AY, Stekhanova TN, Mardanov AV, Ravin NV, Bezsudnova EY, Popov VO, Littlechild JA (2019) Thermostable branched-chain amino acid transaminases from the archaea Geoglobus acetivorans and Archaeoglobus fulgidus: biochemical and structural characterization. Front Bioeng Biotechnol 7:1–16. https://doi.org/10.3389/fbioe.2019.00007
Iwasaki A, Matsumoto K, Hasegawa J, Yasohara Y (2012) A novel transaminase, (R)-amine:pyruvate aminotransferase, from Arthrobacter sp. KNK168 (FERM BP-5228): purification, characterization, and gene cloning. Appl Microbiol Biotechnol 93:1563–1573. https://doi.org/10.1007/s00253-011-3580-0
Iwasaki A, Yamada Y, Kizaki N, Ikenaka Y, Hasegawa J (2006) Microbial synthesis of chiral amines by (R)-specific transamination with Arthrobacter sp. KNK168. Appl Microbiol Biotechnol 69:499–505. https://doi.org/10.1007/s00253-005-0002-1
Jansonius JN (1998) Structure, evolution and action of vitamin B6-dependent enzymes. Curr Opin Struct Biol 8:759–769. https://doi.org/10.1016/S0959-440X(98)80096-1
Jiang J, Chen X, Zhang D, Wu Q, Zhu D (2015) Characterization of (R)-selective amine transaminases identified by in silico motif sequence blast. Appl Microbiol Biotechnol 99:2613–2621. https://doi.org/10.1007/s00253-014-6056-1
John RA (1995) Pyridoxal phosphate-dependent enzymes. Biochim Biophys Acta - Protein Struct Mol Enzymol 1248:81–96. https://doi.org/10.1016/0167-4838(95)00025-P
Kirsch JF, Eichele G, Ford GC, Vincent MG, Jansonius JN, Gehring H, Christen P (1984) Mechanism of action of aspartate aminotransferase proposed on the basis of its spatial structure. J Mol Biol 174:497–525. https://doi.org/10.1016/0022-2836(84)90333-4
Kishimoto K, Yoshimura T, Nobuyoshi E, Shigetoshi S, Manning JM (1995) Role of Leucine 201 of thermostable aminotransferase from a thermophile, Bacillus sp. YM-1. J Biochem 117:691–696
Kobayashi J, Shimizu Y, Mutaguchi Y, Doi K, Ohshima T (2013) Characterization of d-amino acid aminotransferase from Lactobacillus salivarius. J Mol Catal B Enzym 94:15–22. https://doi.org/10.1016/j.molcatb.2013.04.013
Lepore BW, Liu D, Peng Y, Fu M, Yasuda C, Manning JM, Silverman RB, Ringe D (2010) Chiral discrimination among aminotransferases: inactivation by 4-amino-4,5-dihydrothiophenecarboxylic acid. Biochemistry 49:3138–3147. https://doi.org/10.1021/bi902052x
Łyskowski A, Gruber C, Steinkellner G, Schürmann M, Schwab H, Gruber K, Steiner K (2014) Crystal structure of an (R)-selective ω-transaminase from Aspergillus terreus. PLoS One 9:e87350. https://doi.org/10.1371/journal.pone.0087350
Norton JE, Sokatch JR (1970) Purification and partial characterization of the branched chain amino acid transaminase of Pseudomonas aeruginosa. Biochim Biophys Acta - Enzymol 206:261–269. https://doi.org/10.1016/0005-2744(70)90109-9
Okada K, Hirotsu K, Hayashi H, Kagamiyama H (2001) Structures of Escherichia coli branched-chain amino acid aminotransferase and its complexes with 4-methylvalerate and 2-methylleucine: induced fit and substrate recognition of the enzyme. Biochemistry 40:7453–7463. https://doi.org/10.1021/bi010384l
Park E-S, Dong J-Y, Shin J-S (2014) Active site model of (R)-selective ω-transaminase and its application to the production of d-amino acids. Appl Microbiol Biotechnol 98:651–660. https://doi.org/10.1007/s00253-013-4846-5
Park E-S, Kim M, Shin J-S (2010) One-pot conversion of L-threonine into L-homoalanine: biocatalytic production of an unnatural amino acid from a natural one. Adv Synth Catal 352:3391–3398. https://doi.org/10.1002/adsc.201000601
Patil MD, Grogan G, Bommarius A, Yun H (2018) Recent advances in ω-transaminase-mediated biocatalysis for the enantioselective synthesis of chiral amines. Catalysts 8:254. https://doi.org/10.3390/catal8070254
Pavkov-Keller T, Strohmeier GA, Diepold M, Peeters W, Smeets N, Schürmann M, Gruber K, Schwab H, Steiner K (2016) Discovery and structural characterisation of new fold type IV-transaminases exemplify the diversity of this enzyme fold. Sci Rep 6:38183. https://doi.org/10.1038/srep38183
Payer SE, Schrittwieser JH, Kroutil W (2017) Vicinal diamines as smartcosubstrates in the transaminase-catalyzed asymmetric amination of ketones. European J Org Chem 2017:2553–2559. https://doi.org/10.1002/ejoc.201700253
Peisach D, Chipman DM, Van Ophem PW, Manning JM, Ringe D (1998) Crystallographic study of steps along the reaction pathway of D -amino acid aminotransferase. Biochemistry 37:4958–4967. https://doi.org/10.1021/bi972884d
Pucci MJ, Thanassi JA, Ho HT, Falk PJ, Dougherty TJ (1995) Staphylococcus haemolyticus contains two D-glutamic acid biosynthetic activities, a glutamate racemase and a D-amino acid transaminase. J Bacteriol 177:336–342. https://doi.org/10.1128/jb.177.2.336-342.1995
Radkov AD, Moe LA (2014) Bacterial synthesis of d-amino acids. Appl Microbiol Biotechnol 98:5363–5374. https://doi.org/10.1007/s00253-014-5726-3
Rudat J, Brucher BR, Syldatk C (2012) Transaminases for the synthesis of enantiopure beta-amino acids. AMB Express 2:11. https://doi.org/10.1186/2191-0855-2-11
Savile CK, Janey JM, Mundorff EC, Moore JC, Tam S, Jarvis WR, Colbeck JC, Krebber A, Fleitz FJ, Brands J, Devine PN, Huisman GW, Hughes GJ (2010) Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 329:305–309. https://doi.org/10.1126/science.1188934
Sayer C, Martinez-Torres RJ, Richter N, Isupov MN, Hailes HC, Littlechild JA, Ward JM (2014) The substrate specificity, enantioselectivity and structure of the ( R )-selective amine : pyruvate transaminase from Nectria haematococca. FEBS J 281:2240–2253. https://doi.org/10.1111/febs.12778
Schätzle S, Steffen-Munsberg F, Thontowi A, Höhne M, Robins K, Bornscheuer UT (2011) Enzymatic asymmetric synthesis of enantiomerically pure aliphatic, aromatic and arylaliphatic amines with (R)-selective amine transaminases. Adv Synth Catal 353:2439–2445. https://doi.org/10.1002/adsc.201100435
Schneider G, Käck H, Lindqvist Y (2000) The manifold of vitamin B6 dependent enzymes. Structure 8:R1–R6. https://doi.org/10.1016/S0969-2126(00)00085-X
Simon RC, Richter N, Busto E, Kroutil W (2014) Recent developments of cascade reactions involving ω-transaminases. ACS Catal 4:129–143. https://doi.org/10.1021/cs400930v
Skalden L, Thomsen M, Höhne M, Bornscheuer UT, Hinrichs W (2015) Structural and biochemical characterization of the dual substrate recognition of the ( R )-selective amine transaminase from Aspergillus fumigatus. FEBS J 282:407–415. https://doi.org/10.1111/febs.13149
Slabu I, Galman JL, Lloyd RC, Turner NJ (2017) Discovery, engineering, and synthetic application of transaminase biocatalysts. ACS Catal 7:8263–8284. https://doi.org/10.1021/acscatal.7b02686
Steffen-Munsberg F, Vickers C, Kohls H, Land H, Mallin H, Nobili A, Skalden L, van den Bergh T, Joosten H-J, Berglund P, Höhne M, Bornscheuer UT (2015) Bioinformatic analysis of a PLP-dependent enzyme superfamily suitable for biocatalytic applications. Biotechnol Adv 33:566–604. https://doi.org/10.1016/j.biotechadv.2014.12.012
Steffen-Munsberg F, Vickers C, Thontowi A, Schätzle S, Meinhardt T, Svedendahl Humble M, Land H, Berglund P, Bornscheuer UT, Höhne M (2013) Revealing the structural basis of promiscuous amine transaminase activity. ChemCatChem 5:154–157. https://doi.org/10.1002/cctc.201200545
Stekhanova TN, Rakitin AL, Mardanov AV, Bezsudnova EY, Popov VO (2017) A novel highly thermostable branched-chain amino acid aminotransferase from the crenarchaeon Vulcanisaeta moutnovskia. Enzym Microb Technol 96:127–134. https://doi.org/10.1016/j.enzmictec.2016.10.002
Sugio S, Petsko GA, Manning JM, Soda K, Ringe D (1995) Crystal structure of a D-amino acid aminotransferase: how the protein controls stereoselectivity. Biochemistry 34:9661–9669. https://doi.org/10.1021/bi00030a002
Tanizawa K, Masu Y, Asano S, Tanaka H, Soda K (1989) Thermostable D-amino acid aminotransferase from a thermophilic Bacillus species. J Biol Chem 264:2445–2449
Telzerow A, Paris J, Håkansson M, González-Sabín J, Ríos-Lombardía N, Schürmann M, Gröger H, Morís F, Kourist R, Schwab H, Steiner K (2019) Amine transaminase from Exophiala Xenobiotica —crystal structure and engineering of a fold IV transaminase that naturally converts biaryl ketones. ACS Catal 9:1140–1148. https://doi.org/10.1021/acscatal.8b04524
Thomsen M, Skalden L, Palm GJ, Höhne M, Bornscheuer UT, Hinrichs W (2014) Crystallographic characterization of the ( R )-selective amine transaminase from Aspergillus fumigatus. Acta Crystallogr Sect D Biol Crystallogr 70:1086–1093. https://doi.org/10.1107/S1399004714001084
Toney MD (2011) Controlling reaction specificity in pyridoxal phosphate enzymes. Biochim Biophys Acta - Proteins Proteomics 1814:1407–1418. https://doi.org/10.1016/j.bbapap.2011.05.019
Toney MD, Kirsch JF (1991) The K258R mutant of aspartate aminotransferase stabilizes the quinonoid intermediate. J Biol Chem 266:23900–23903
Tufvesson P, Jensen JS, Kroutil W, Woodley JM (2012) Experimental determination of thermodynamic equilibrium in biocatalytic transamination. Biotechnol Bioeng 109:2159–2162. https://doi.org/10.1002/bit.24472
Uchida Y, Hayashi H, Washio T, Yamasaki R, Kato S, Oikawa T (2014) Cloning and characterization of a novel fold-type I branched-chain amino acid aminotransferase from the hyperthermophilic archaeon Thermococcus sp. CKU-1. Extremophiles 18:589–602. https://doi.org/10.1007/s00792-014-0642-0
Venos ES, Knodel MH, Radford CL, Berger BJ (2004) Branched-chain amino acid aminotransferase and methionine formation in Mycobacterium tuberculosis. BMC Microbiol 4:1–14. https://doi.org/10.1186/1471-2180-4-39
Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414:562–565. https://doi.org/10.1038/35107108
Wybenga GG, Crismaru CG, Janssen DB, Dijkstra BW (2012) Structural determinants of the β-selectivity of a bacterial aminotransferase. J Biol Chem 287:28495–28502. https://doi.org/10.1074/jbc.M112.375238
Xian M, Alaux S, Sagot E, Gefflaut T (2007) Chemoenzymatic synthesis of glutamic acid analogues: substrate specificity and synthetic applications of branched chain aminotransferase from Escherichia coli. J Org Chem 72:7560–7566. https://doi.org/10.1021/jo070805q
Xing RY, Whitman WB (1992) Characterization of amino acid aminotransferases of Methanococcus aeolicus. J. Bacteriol., 174, 541–548. https://doi.org/10.1128/jb.174.2.541-548.1992
Yoon S, Patil MD, Sarak S, Jeon H, Kim G, Khobragade TP, Sung S, Yun H, (2019) Deracemization of racemic amines to enantiopure (R )‐ and (S )‐amines by biocatalytic cascade employing ω‐Transaminase and amine dehydrogenase . ChemCatChem 11 (7):1898–1902. https://doi.org/10.1002/cctc.201900080
Yu X, Wang X, Engel PC (2014) The specificity and kinetic mechanism of branched-chain amino acid aminotransferase from Escherichia coli studied with a new improved coupled assay procedure and the enzyme’s potential for biocatalysis. FEBS J 281:391–400. https://doi.org/10.1111/febs.12609
Zaitseva J, Lu J, Olechoski KL, Lamb AL (2006) Two crystal structures of the isochorismate pyruvate lyase from Pseudomonas aeruginosa. J Biol Chem 281:33441–33449. https://doi.org/10.1074/jbc.M605470200
Zeifman YS, Boyko KM, Nikolaeva AY, Timofeev VI, Rakitina TV, Popov VO, Bezsudnova EY (2019) Functional characterization of PLP fold type IV transaminase with a mixed type of activity from Haliangium ochraceum. Biochim Biophys Acta - Proteins Proteomics 1867:575–585. https://doi.org/10.1016/j.bbapap.2019.03.005
Funding
The analysis of the structural features of R-TAs and TAs with an expanded substrate specificity was supported by the Russian Science Foundation project № 19-14-00164. The analysis of BCATs was supported by Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 192 kb)
Rights and permissions
About this article
Cite this article
Bezsudnova, E.Y., Popov, V.O. & Boyko, K.M. Structural insight into the substrate specificity of PLP fold type IV transaminases. Appl Microbiol Biotechnol 104, 2343–2357 (2020). https://doi.org/10.1007/s00253-020-10369-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10369-6