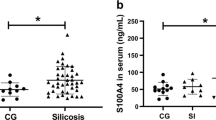
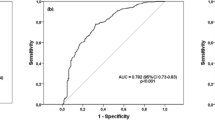
Abstract
Silicosis is one of the prolonged and irreversible occupational diseases. Crystalline silica dust, which has been linked with silicosis, occurs in different industrial areas such as constructions, ceramic, quarry, and pottery. There are significant numbers of newly diagnosed cases every year in Turkey. Patients with silicosis suffer from inflammatory respiratory disorders and silicosis-related complications such as rheumatoid arthritis, systemic sclerosis, and vasculitis. Oxysterols are defined as 27-carbon intermediates or end products of cholesterol. They are also implicated in the etiology of disease states such as atherosclerosis, neurodegenerative, and inflammatory diseases. The aim of the study is to evaluate cholesterol oxidation products in the patients with silicosis and determination of sphingosine-1-phosphate (S1P) levels which is a sphingolipid metabolite. In addition to these parameters, it is aimed to determine the possible lipid peroxidation by different parameters. For this purpose, blood samples and urine were collected from 47 patients and 30 healthy individual with their consents. In order to evaluate oxysterols, 7-ketocholesterol and cholestan 3β,5α,6β-triol levels were measured by LC–MS/MS method. The measured levels of 7-KC were 0.101 ± 0.005 µmol/l in patient and 0.050 ± 0.003 µmol/l in control plasma samples. Triol levels were measured as 0.038 ± 0.005 µmol/l in patient group and 0.033 ± 0.004 µmol/l in control group (p < 0.001). In addition, lipid peroxidation products were measured by human-8-isoprostane, human-4-hydroxynonenal (4-HNE), and human malondialdehyde (MDA) ELISA kits. The measured levels of HNE in the patient and control groups were 735.14 ± 288.80 pg/ml and 595.72 ± 108.62 pg/ml in plasma and 606.02 + 118.23 pg/ml and 531.84 + 107.18 pg/ml in urine, respectively (p < 0.05). F2-iP results of patients and controls were 450.0 + 101.40 pg/dl and 386.9 + 112.7 pg/ml for urine and 432.7 ± 188,8 pg/dl and 321.9 ± 69.4 pg/dl for plasma, respectively (p < 0.05). MDA levels of plasma were measured as 44.1 ± 14.6 nmol/ml in the patient and 31.9 ± 10.5 nmol/ml in the control (p < 0.05). Levels of MDA for urine samples were 30.15 + 5.06 nmol/ml and 25.15 + 6.07 nmol/ml in patients and controls, respectively (p < 0.05). S1P levels were decreased in patients compared to control group (49.05 ± 10.87 and 67.57 ± 16.25, p < 0.001). The results not only indicate a correlation between cholesterol oxidation, lipid peroxidation, and silicosis, but also provide better understanding of the role of the lipids in the mechanism of this inflammatory disease.





Similar content being viewed by others
References
Prasad B, Pandey J (2016) Serum copper and zinc level as biomarker for dust exposed lung diseases among coal miners. J Biodiv Environ Sci 8:65–74
Pandey JK, Agarwal D (2012) Biomarkers: a potential prognostic tool for silicosis. Indian J Occup environmental Med 16:101
Karkhanis VS, Joshi J (2012) Combined pulmonary fibrosis and emphysema in a tyre industry worker. Lung India 29:273
Akgun M, Araz O, Akkurt I, Eroglu A, Alper F, Saglam L, Mirici A, Gorguner M, Nemery B (2008) An epidemic of silicosis among former denim sandblasters. Eur Respir J 32:1295–1303
Anlar HG, Bacanli M, İritaş S, Bal C, Kurt T, Tutkun E, Hinc Yilmaz O, Basaran N (2017) Effects of occupational silica exposure on oxidative stress and immune system parameters in ceramic workers in Turkey. J Toxicol Environ Health Part A 80:688–696
Akgün M, Ergan B (2018) Silicosis in Turkey: is it an endless nightmare or is there still hope? Turkish Thorac J 19:89
Archontogeorgis K, Steiropoulos P, Tzouvelekis A, Nena E, Bouros D (2012) Lung cancer and interstitial lung diseases: a systematic review. Pulm Med. https://doi.org/10.1155/2012/315918
Sabuncuoğlu S, Öztaş Y (2014) Oxysterols and their metabolic roles beyond cholesterol: a reappraisal. Acta Med 45:75–79
Bargagli E, Olivieri C, Bennett D, Prasse A, Muller-Quernheim J, Rottoli P (2009) Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med 103:1245–1256
Vallyathan V, Leonard S, Kuppusamy P, Pack D, Chzhan M, Sanders SP, Zweir JL (1997) Oxidative stress in silicosis: evidence for the enhanced clearance of free radicals from whole lungs. Mol Cell Biochem 168:125–132
Shi X, Mao Y, Daniel LN, Saffiotti U, Dalal NS, Vallyathan V (1994) Silica radical-induced DNA damage and lipid peroxidation. Environ Health Perspect 102(10):149–154. https://doi.org/10.1289/ehp.94102s10149
Nardi J, Nascimento S, Goethel G, Gauer B, Sauer E, Fao N, Cestonaro L, Peruzzi C, Souza J, Garcia SC (2018) Inflammatory and oxidative stress parameters as potential early biomarkers for silicosis. Clin Chim Acta 484:305–313. https://doi.org/10.1016/j.cca.2018.05.045
Luu W, Sharpe LJ, Capell-Hattam I, Gelissen IC, Brown AJ (2016) Oxysterols: old tale, new twists. Annu Rev Pharmacol Toxicol 56:447–467
Lütjohann D, Lizard G, Iuliano L (2017) Oxysterols: players in different metabolic leagues. J Steroid Biochem Mol Biol. https://doi.org/10.1016/j.jsbmb.2017.03.011
Iuliano L (2011) Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem Phys Lipid 164:457–468
Niki E (2008) Lipid peroxidation products as oxidative stress biomarkers. BioFactors 34:171–180
Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22:50–60. https://doi.org/10.1016/j.tcb.2011.09.003
Rimal B, Greenberg AK, Rom WN (2005) Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med 11:169–173
Jiang X, Sidhu R, Porter FD, Yanjanin NM, Speak AO, te Vruchte DT, Platt FM, Fujiwara H, Scherrer DE, Zhang J (2011) A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J Lipid Res 52:1435–1445
Zhang Y, Berka V, Song A, Sun K, Wang W, Zhang W, Ning C, Li C, Zhang Q, Bogdanov M, Alexander DC, Milburn MV, Ahmed MH, Lin H, Idowu M, Zhang J, Kato GJ, Abdulmalik OY, Zhang W, Dowhan W, Kellems RE, Zhang P, Jin J, Safo M, Tsai AL, Juneja HS, Xia Y (2014) Elevated sphingosine-1-phosphate promotes sickling and sickle cell disease progression. J Clin Invest 124:2750–2761. https://doi.org/10.1172/JCI74604
Sari G, Simsek C, Gebesoglu B, Gulgosteren S, Uzmezoglu B and Celik D (2017) Accelerated silicosis in teflon-coated pan manufacturing: case report. C59. SILICOSIS, American Thoracic Society, pp. A5963–A5963
Köksal N, Kahraman H (2011) Acute silicosis in teflon-coated pan manufacturing due to metal sandblasting. Int J Occup Environ Health 17:210–213
Thomas CR, Kelley TR (2010) A brief review of silicosis in the United States. Environ Health Insights 4:EHI-S4628
Peluso ME, Munnia A, Giese RW, Chellini E, Ceppi M, Capacci F (2015) Oxidatively damaged DNA in the nasal epithelium of workers occupationally exposed to silica dust in Tuscany region, Italy. Mutagenesis 30:519–525
Wang Y, Yang G, Zhu Z, Liang D, Niu P, Gao A, Chen L, Tian L (2015) Effect of bone morphogenic protein-7 on the expression of epithelial–mesenchymal transition markers in silicosis model. Exp Mol Pathol 98:393–402
Ikonen E (2008) Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol 9:125–138. https://doi.org/10.1038/nrm2336
Berkowitz ML (2009) Detailed molecular dynamics simulations of model biological membranes containing cholesterol. Biochim Biophys Acta (BBA) 1788:86–96
Kulig W, Cwiklik L, Jurkiewicz P, Rog T, Vattulainen I (2016) Cholesterol oxidation products and their biological importance. Chem Phys Lipid 199:144–160. https://doi.org/10.1016/j.chemphyslip.2016.03.001
Zarrouk A, Vejux A, Mackrill J, O’Callaghan Y, Hammami M, O’Brien N, Lizard G (2014) Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res Rev 18:148–162
Cojocaru M, Niculescu T (1997) Study of plasma lipid peroxides in silicosis. Jugoslovenska Medicinska Biohemija-Yugoslav Med Biochem 16:25–27
Syslova K, Kacer P, Kuzma M, Najmanova V, Fenclova Z, Vlckova S, Lebedova J, Pelclova D (2009) Rapid and easy method for monitoring oxidative stress markers in body fluids of patients with asbestos or silica-induced lung diseases. J Chromatogr B 877:2477–2486. https://doi.org/10.1016/j.jchromb.2009.06.008
Pelclova D, Fenclova Z, Syslova K, Vlckova S, Lebedova J, Pecha O, Belacek J, Navratil T, Kuzma M, Kacer P (2011) Oxidative stress markers in exhaled breath condensate in lung fibroses are not significantly affected by systemic diseases. Ind Health 49:746–754
Arezzini B, Vecchio D, Signorini C, Stringa B, Gardi C (2018) F2-isoprostanes can mediate bleomycin-induced lung fibrosis. Free Radic Biol Med 115:1–9. https://doi.org/10.1016/j.freeradbiomed.2017.11.007
Montuschi P, Ciabattoni G, Paredi P, Pantelidis P, du Bois RM, Kharitonov SA, Barnes PJ (1998) 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am J Respir Crit Care Med 158:1524–1527. https://doi.org/10.1164/ajrccm.158.5.9803102
Muus P, Bonta IL, den Oudsten SA (1979) Plasma levels of malondialdehyde, a product of cyclo-oxygenase-dependent and independent lipid peroxidation in rheumatoid arthritis: a correlation with disease activity. Prostaglandins Med 2:63–65
Bragt PC, Schenkelaars EP, Bonta IL (1979) Dissociation between prostaglandin and malondialdehyde formation in exudate and increased levels of malondialdehyde in plasma and liver during granulomatous inflammation in the rat. Prostaglandins Med 2:51–61
Zhang H, Yin G, Jiang H, Zhang C (2013) High-dose N-acetylcysteine decreases silica-induced lung fibrosis in the rat. J Int Med Res 41:1179–1186. https://doi.org/10.1177/0300060513488503
Azari M, Ramazani B, Ali Mosavian M, Movahadi M, Salehpour S (2011) Serum malondialdehyde and urinary neopterin levels in glass sandblasters exposed to crystalline silica aerosols. Int J Occup Hyg 3(1):29–32
Anlar HG, Bacanli M, Iritas S, Bal C, Kurt T, Tutkun E, Hinc Yilmaz O, Basaran N (2017) Effects of occupational silica exposure on oxidative stress and immune system parameters in ceramic workers in Turkey. J Toxicol Environ Health A 80:688–696. https://doi.org/10.1080/15287394.2017.1286923
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Barrera G, Pizzimenti S, Ciamporcero ES, Daga M, Ullio C, Arcaro A, Cetrangolo GP, Ferretti C, Dianzani C, Lepore A, Gentile F (2015) Role of 4-hydroxynonenal-protein adducts in human diseases. Antioxid Redox Signal 22:1681–1702. https://doi.org/10.1089/ars.2014.6166
Barrera G, Pizzimenti S, Daga M, Dianzani C, Arcaro A, Cetrangolo GP, Giordano G, Cucci MA, Graf M, Gentile F (2018) Lipid Peroxidation-derived aldehydes, 4-hydroxynonenal and malondialdehyde in aging-related disorders. Antioxidants (Basel). https://doi.org/10.3390/antiox7080102
Nikolaidis MG, Kyparos A, Vrabas IS (2011) F2-isoprostane formation, measurement and interpretation: the role of exercise. Prog Lipid Res 50:89–103
Czerska M, Zielinski M and Gromadzinska J (2015) Isoprostanes: a novel major group of oxidative stress markers Biochim Biophys Acta, 1851(4), 433–445.
Pelclova D, Fenclova Z, Kacer P, Navratil T, Kuzma M, Lebedova JK, Klusackova P (2007) 8-isoprostane and leukotrienes in exhaled breath condensate in Czech subjects with silicosis. Ind Health 45:766–774
Björkhem I, Diczfalusy U, Lütjohann D (1999) Removal of cholesterol from extrahepatic sources by oxidative mechanisms. Curr Opin Lipidol 10:161–165
Leonarduzzi G, Sottero B, Poli G (2002) Oxidized products of cholesterol: dietary and metabolic origin, and proatherosclerotic effects. J Nutr Biochem 13:700–710
van Reyk DM, Brown AJ, Hult'en LM, Dean RT, Jessup W (2006) Oxysterols in biological systems: sources, metabolism and pathophysiological relevance. Redox Rep 11:255–262. https://doi.org/10.1179/135100006X155003
Jusakul A, Yongvanit P, Loilome W, Namwat N, Kuver R (2011) Mechanisms of oxysterol-induced carcinogenesis. Lipids Health Dis. https://doi.org/10.1186/1476-511x-10-44
Poli G, Biasi F, Leonarduzzi G (2013) Oxysterols in the pathogenesis of major chronic diseases. Redox Biol 1:125–130
Vejux A, Lizard G (2009) Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol Aspects Med 30:153–170
Hinamoto N, Maeshima Y, Saito D, Yamasaki H, Tanabe K, Nasu T, Watatani H, Ujike H, Kinomura M, Sugiyama H (2014) Urinary and plasma levels of vasohibin-1 can predict renal functional deterioration in patients with renal disorders. PLoS ONE 9:e96932
Nury T, Zarrouk A, Ragot K, Debbabi M, Riedinger J-M, Vejux A, Aubourg P, Lizard G (2017) 7-Ketocholesterol is increased in the plasma of X-ALD patients and induces peroxisomal modifications in microglial cells: Potential roles of 7-ketocholesterol in the pathophysiology of X-ALD. J Steroid Biochem Mol Biol 169:123–136
Acknowledgements
The authors are thankful to all study participants. The authors would like to acknowledge all colleagues who assisted with this study. This study was supported by Hacettepe University Coordination Unit for Scientific Research Projects (GO 16/697-18).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aksu, N., Samadi, A., Yalçınkaya, A. et al. Evaluation of oxysterol levels of patients with silicosis by LC–MS/MS method. Mol Cell Biochem 467, 117–125 (2020). https://doi.org/10.1007/s11010-020-03706-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03706-w