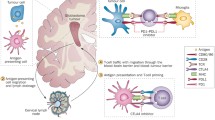
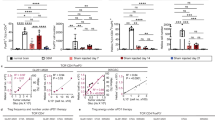
Abstract
Purpose
Immunotherapy, activation of the immune system to target tumor cells, represents a paradigm shift in the treatment of cancer. Immune checkpoint therapies, which target immunomodulatory molecules expressed on T-lymphocytes, have demonstrated improved survival in a variety of malignancies. However, benefit in glioblastoma, the most common and devastating malignant brain tumor, remains to be seen. With several recent clinical trials failing to show efficacy of immunotherapy, concerns have been raised regarding the impact of glucocorticoid use in this patient population that may impair the ability for immune checkpoint inhibitors to affect a response.
Methods
For this article we examined the mechanism by which immune checkpoint inhibitors activate, and glucocorticoids impair, T-lymphocyte function.
Results
In this context, we review the clinical data of immune checkpoint inhibitors in glioblastoma as well as the impact glucocorticoids have on immune checkpoint inhibitor efficacy. Finally, we highlight key questions that remain in the field, and the potential benefit of further research for central nervous system tumors.
Conclusion
More information on the extent, character and duration of glucocorticoids on patients treated with PD-(L)1 will better inform both clinical management and novel therapeutic development of immunotherapy in patients with CNS malignancies.


Similar content being viewed by others
References
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D et al (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1116–1127
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD et al (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1):23–34
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379(22):2108–2121
Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL et al (2018) Nivolumab for relapsed/refractory classic hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 Trial. J Clin Oncol 36(14):1428–1439
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H et al (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192(7):1027–1034
Jacobs JF, Idema AJ, Bol KF, Nierkens S, Grauer OM, Wesseling P et al (2009) Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol 11(4):394–402
Krummel MF, Allison JP (1995) CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 182(2):459–465
Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z et al (2008) CTLA-4 control over Foxp3 + regulatory T cell function. Science 322(5899):271–275
Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP (1992) CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 356(6370):607–609
Omuro A, Vlahovic G, Lim M, Sahebjam S, Baehring J, Cloughesy T et al (2018) Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol 20(5):674–686
Reardon DAOA, Brandes AA et al (2017) OS10.3 randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro-Oncology 1(19(3):iii21
Bristol-Myers Squibb Provides Update on Phase 3 Opdivo (nivolumab) CheckMate—548 trial in patients with newly diagnosed MGMT-methylated glioblastoma multiforme. https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-provides-update-phase-3-opdivo-nivolumab. Accessed 10 Dec 2019
Bristol-Myers Squibb Announces Phase 3 CheckMate – 498 Study Did Not Meet Primary Endpoint of Overall Survival with Opdivo (niovlumab) Plus Radiation in Patients with Newly Diagnosed MGMT-Unmethylated Glioblastoma Multifrome. https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-phase-3-checkmate-498-study-did. Accessed 10 Dec 2019
Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB et al (2019) Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 25(3):477–486
Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, Lopez-Janeiro A, Porciuncula A, Idoate MA et al (2019) Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med 25(3):470–476
Zhao J, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T et al (2019) Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med 25(3):462–469
Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M et al (2016) MAP Kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity 44(3):609–621
Hapgood JP, Avenant C, Moliki JM (2016) Glucocorticoid-independent modulation of GR activity: implications for immunotherapy. Pharmacol Ther 165:93–113
De Bosscher K, Schmitz ML, Vanden Berghe W, Plaisance S, Fiers W, Haegeman G (1997) Glucocorticoid-mediated repression of nuclear factor-kappaB-dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci USA 94(25):13504–13509
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM et al (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36(17):1714–1768
Dexamethasone Tablets. https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/11664slr062_decadron_lbl.pdf. Accessed 27 Oct 2019
Galicich JH, French LA, Melby JC (1961) Use of dexamethasone in treatment of cerebral edema associated with brain tumors. J Lancet 81:46–53
Czock D, Keller F, Rasche FM, Haussler U (2005) Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 44(1):61–98
Loew D, Schuster O, Graul EH (1986) Dose-dependent pharmacokinetics of dexamethasone. Eur J Clin Pharmacol 30(2):225–230
Spoorenberg SM, Deneer VH, Grutters JC, Pulles AE, Voorn GP, Rijkers GT et al (2014) Pharmacokinetics of oral vs. intravenous dexamethasone in patients hospitalized with community-acquired pneumonia. Br J Clin Pharmacol 78(1):78–83
Weissman DE, Janjan NA, Erickson B, Wilson FJ, Greenberg M, Ritch PS et al (1991) Twice-daily tapering dexamethasone treatment during cranial radiation for newly diagnosed brain metastases. J Neurooncol 11(3):235–239
Pitter KL, Tamagno I, Alikhanyan K, Hosni-Ahmed A, Pattwell SS, Donnola S et al (2016) Corticosteroids compromise survival in glioblastoma. Brain 139(Pt 5):1458–1471
Wong ET, Lok E, Gautam S, Swanson KD (2015) Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br J Cancer 113(2):232–241
Hughes MA, Parisi M, Grossman S, Kleinberg L (2005) Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys 62(5):1423–1426
Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A et al (2015) Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-Oncology 17(8):1064–1075
Berghoff AS, Fuchs E, Ricken G, Mlecnik B, Bindea G, Spanberger T et al (2016) Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology 5(1):e1057388
Giles AJ, Hutchinson MND, Sonnemann HM, Jung J, Fecci PE, Ratnam NM et al (2018) Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer 6(1):51
Fuca G, Galli G, Poggi M, Lo Russo G, Proto C, Imbimbo M et al (2019) Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 4(1):e000457
Cohen JJ (1972) Thymus-derived lymphocytes sequestered in the bone marrow of hydrocortisone-treated mice. J Immunol 108(3):841–844
Bloemena E, Weinreich S, Schellekens PT (1990) The influence of prednisolone on the recirculation of peripheral blood lymphocytes in vivo. Clin Exp Immunol 80(3):460–466
Van Laethem F, Baus E, Smyth LA, Andris F, Bex F, Urbain J et al (2001) Glucocorticoids attenuate T cell receptor signaling. J Exp Med 193(7):803–814
Lowenberg M, Tuynman J, Bilderbeek J, Gaber T, Buttgereit F, van Deventer S et al (2005) Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood 106(5):1703–1710
Marchetti MC, Di Marco B, Cifone G, Migliorati G, Riccardi C (2003) Dexamethasone-induced apoptosis of thymocytes: role of glucocorticoid receptor-associated Src kinase and caspase-8 activation. Blood 101(2):585–593
Northrop JP, Crabtree GR, Mattila PS (1992) Negative regulation of interleukin 2 transcription by the glucocorticoid receptor. J Exp Med 175(5):1235–1245
Paliogianni F, Raptis A, Ahuja SS, Najjar SM, Boumpas DT (1993) Negative transcriptional regulation of human interleukin 2 (IL-2) gene by glucocorticoids through interference with nuclear transcription factors AP-1 and NF-AT. J Clin Invest 91(4):1481–1489
Nijhuis EW, Hinloopen B, van Lier RA, Nagelkerken L (1995) Differential sensitivity of human naive and memory CD4 + T cells for dexamethasone. Int Immunol 7(4):591–595
Lanza L, Scudeletti M, Puppo F, Bosco O, Peirano L, Filaci G et al (1996) Prednisone increases apoptosis in in vitro activated human peripheral blood T lymphocytes. Clin Exp Immunol 103(3):482–490
Olnes MJ, Kotliarov Y, Biancotto A, Cheung F, Chen J, Shi R et al (2016) Effects of systemically administered hydrocortisone on the human immunome. Sci Rep 6:23002
Xia M, Gasser J, Feige U (1999) Dexamethasone enhances CTLA-4 expression during T cell activation. Cell Mol Life Sci 55(12):1649–1656
Xing K, Gu B, Zhang P, Wu X (2015) Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: an insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunol 16:39
Harmankaya K, Erasim C, Koelblinger C, Ibrahim R, Hoos A, Pehamberger H et al (2011) Continuous systemic corticosteroids do not affect the ongoing regression of metastatic melanoma for more than two years following ipilimumab therapy. Med Oncol 28(4):1140–1144
Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK et al (2015) Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 33(28):3193–3198
Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC et al (2005) Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 23(25):6043–6053
Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE et al (2007) Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res 13(22 Pt 1):6681–6688
Garant A, Guilbault C, Ekmekjian T, Greenwald Z, Murgoi P, Vuong T (2017) Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: a systematic review. Crit Rev Oncol Hematol 120:86–92
Parakh S, Park JJ, Mendis S, Rai R, Xu W, Lo S et al (2017) Efficacy of anti-PD-1 therapy in patients with melanoma brain metastases. Br J Cancer 116(12):1558–1563
Queirolo P, Spagnolo F, Ascierto PA, Simeone E, Marchetti P, Scoppola A et al (2014) Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol 118(1):109–116
Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I et al (2012) Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 13(5):459–465
Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A et al (2018) Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 36(28):2872–2878
Della Corte CM, Morgillo F (2019) Early use of steroids affects immune cells and impairs immunotherapy efficacy. ESMO Open 4(1):e000477
Banna GL, Passiglia F, Colonese F, Canova S, Menis J, Addeo A et al (2018) Immune-checkpoint inhibitors in non-small cell lung cancer: a tool to improve patients’ selection. Crit Rev Oncol Hematol 129:27–39
Funding
No financial support was provided for this review.
Author information
Authors and Affiliations
Contributions
WJK performed the literature search and drafted the initial manuscript. MRG provided concept and study interpretation. All authors contributed to manuscript editing and have approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
WJK and MRG declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kelly, W.J., Gilbert, M.R. Glucocorticoids and immune checkpoint inhibitors in glioblastoma. J Neurooncol 151, 13–20 (2021). https://doi.org/10.1007/s11060-020-03439-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03439-2