Abstract
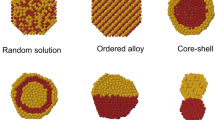
The structure evolution behavior of M-Al (M = Mg, Ni, and Fe) nanoalloys with three sizes (N = 13, 55, and 147) at different compositions were studied through basin-hopping Monte Carlo and molecular dynamics simulations. The atomic interaction was described using the embedded atom method. Results showed that the particle size and composition ratio of each element affected the final configuration of nanoparticles mainly due to the competition between the alloying effect and surface segregation. Among three different systems, the Ni-Al system had the strongest alloying effect, followed by Fe-Al and Mg-Al systems. Among three types of nanoalloys with 13 atoms, the icosahedra (ICO) structure had the most stable configuration. For nanoalloys with 55 and 147 atoms, poly-icosahedral (pIh), three-layer onion-like, rhombohedra (RHO), and core-shell structures were observed by controlling the composition ratio of two different metals. This study can provide theoretical guidance for the laboratory synthesis of nanoalloys with different structures.









Similar content being viewed by others
References
Akbarzadeh H, Abbaspour M, Mehrjouei E (2017) Compiettion between stability of icosahedral and cuboctahedral morphologies in bimetallic nanoalloys. Phys Chem Chem Phys 19:14659–14670
Akbarzadeh H, Mehrjouei E, Shamkhali AN, Abbaspour M, Salemi S, Kamrahi M (2018) Au-Fe nanoparticles visited by MD simulation: structural and thermodynamic properties affected by chemical composition. J Chem 42:9666–9675
Asadi E, Zaeem MA, Nouranian S, Baskes MI (2015) Two-phase solid-liquid coexistence of Ni, Cu, and Al by molecular dynamics simulations using the modified embedded-atom method. Acta Mater 86:169–181
Ashcroft NW, Mermin ND (1976) Solid state physics. Saunders College, Philadelphia
Baletto F, Ferrando R (2005) Structural properties of nanoclusters: energetic, thermodynamic, and kinetic effects. Rev Mod Phys 77:371–423
Baletto F, Mottet C, Ferrando R (2003) Growth of three-shell onionlike bimetallic nanoparticles. Rev Rev Lett 90(13):135504
Barcaro G, Sementa L, Fortunelli A (2016). In: Bhushan B (ed) Nanoalloy simulation. Springer, Netherlands
Barrett CS, Massalski TB (1966) Structure of metals. McGraw- Hill, New York
Bochicchio D, Ferrando R (2013) Morphological instability of core-shell metallic nanoparticles. Phys Rev B 87(16):165435
Calov F (2015) Thermodynamics of nanoalloys. Phys Chem Chem Phys 17:27922–27939
Cerbelaud M, Ferrando R, Barcaro G, Fortunelli A (2011) Optimization of chemical ordering in AgAu nanoalloys. Phys Chem Chem Phys 13:10232–10240
Clercq AD, Giorgio S, Mottet C (2016) Pd surface and Pt subsurface segregation in Pt1−cPdc nanoalloys. J Phys: Condens Matter 28:064006
Darby S, Mortimer-Jones TV, Johnston RL, Roberts C (2002) Theoretical study of Cu-Au nanoalloy clusters using a genetic algorithm. J Chem Phys 116(4):1536–1550
Faken D, Jónsson H (1994) Systematic analysis of local atomic structure combined with 3D computer graphics. Comput Mater Sci 2:279–286
Ferrando R (2012) Computational methods for predicting the structures of nanoalloys. Springer Alloyeau D (ed)
Ferrando R (2013) Symmetry breaking and morphological instabilities in core-shell metallic nanoparticles. J Phys Condens Matter 27:013003
Ferrando R, Fortunelli A, Rossi G (2005) Quantum effects on the structure of pure and binary metallic nanoclusters. Phys Rev B 92(8):085448
Ferrando R, Jellinek J, Johnston RL (2008a) Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev 108:845–910
Ferrando R, Fortunelli A, Johnston RL (2008b) Searching for the optimum structures of alloy nanoclusters. Phys Chem Chem Phys 10:640–649
Fu CL, Ye YY, Yoo MH, Ho KM (1993) Equilibrium point defects in intermetallics with the B2 structure: NiAl and FeAl. Phys Rev B 48(9):6712–6715
Goh J, Akola J, Ferrando R (2017) Geometric structure and chemical ordering of large AuCu clusters: a computational study. J Phys Chem C 121:10809–10816
Guedes-Sobrinho D, Nomiyama RK, Chaves AS, Piotrowski MJ, Silva JLFD (2015) Structure, electronic, and magnetic properties of binary PtnTm55−n (Tm = Fe, Co, Ni, Cu, Zn) nanoclusters: a density functional theory investigation. J Phys Chem C 119:15669–15679
Haftel MI (1993) Surface rreconstruction of platinum and gold and the embedded-atom model. Phys Rev B 48:2611–2622
Hultgren R, Desai PD, Hawkins DT, Gleiser M, Kelley KK, Wagman DD (1973) Selected values of the thermodynamic properties of binary alloys. American Society for Metals, Metals Park
Jelinek B, Groh S, Horstemeyer MF, Houze J, Kim SG, Wagner GJ, Moitra A, Baskes MI (2012) Modified embedded atom method potential for Al, Si, Mg, Cu, and Fe alloys. Phys Rev B 85:245102
Kim Y, Kim NJ, Lee B-J (2009) Atomistic modeling of pure Mg and Mg-Al systems. CalPhad 33:650–657
Langlois C, Li ZL, Yuan J, Alloyeau D, Nelayah J, Bochicchio D, Ferrando R, Ricolleau C (2012) Transition from core-shell to Janus chemical configuration for bimetallic nanoparticles. Nanoscale 4:3381–3388
Lee E, Lee B-J (2010) Modified embedded-atom method interatomic potential for the Fe-Al system. J Phys: Condens Matter 22:175702
Li M, Cheng D (2013) Molecular dynamics simulation of the melting behavior of crown-jewel structured Au-Pd nanoalloys. J Phys Chem C 117:18746–18751
Los JH, Mottet C, Tréglia G (2013) Surface segregation trends in transition metal alloys. Phys Rev B 88:165408
Lu J, Ishimura K, Sakaki S (2018) Theoretical insight into core-shell preference for bimetallic Pt-M (M = Ru, Rh, Os, and Ir) cluster and its electronic structure. J Phys Chem C 122:9081–9090
Marks LD, Peng L (2016) Nanoparticle shape, thermodynamics and kinetics. J Phys: Condens Matter 28:053001
Mishin Y, Mehl MJ, Papaconstantopoulos DA (2002) Embedded-atom potential for B2-NiAl. Phys Rev B 65:224114
Narasimhan S, Davenport JW (1995) Ab initio study of polytetrahedral packing: the Al-Mg system. Phys Rev B 51:659–662
Panizon E, Ferrando R (2015) Solid-solid transitions in Pd-Pt nanoalloys. Phys Rev B 92:205417
Pavan L, Baletto F, Novakovic R (2015) Multiscale approach for studying melting transitions in CuPt nanoparticles. Phys Chem Chem Phys 17:28364–28371
Rapetti D, Ferrando R (2019) Density functional theory global optimization of chemical ordering in AgAu nanoalloys. J Alloy Comp 779:582–589
Rapallo A, Rossi G, Ferrando R, Fortunelli A, Curley BC, Tarbuck LDLGM, Johnston RL (2005a) Global optimization of bimetallic cluster structures I. Size-mismatched Ag-Cu, Ag-Ni, and Au-Cu systems. J Chem Phys 122:194308
Rapallo A, Rossi G, Ferrando R, Fortunelli A, Curley BC, Tarbuck LDLGM, Johnston RL (2005b) Global optimization of bimetallic cluster structures II. Size-mismatched Ag-Pd, Ag-Au, and Pd-Pt systems. J Chem Phys 122:194309
Rexer EF, Jellinek, Krissinel EB, Parks EK, Riley SJ (2002) Theoretical and experimental studies of the structures of 12-, 13-, and 14-atom bimetallic nickel/aluminum clusters. J Chem Phys 117 (1):82–94
Rondina GG, Silva JLFD (2013) Revised basin-hopping Monte Carlo algorithm for structure optimization of clusters and nanoparticles. J Chem Inf Model 53:2282–2298
Rossi K, Baletto F (2017) The effect of chemical ordering and lattice mismatch on structural transitions in phase segregating nanoalloys. Phys Chem Chem Phys 19:11057–11063
Rossi G, Ferrando R (2009) Searching for low-energy structures of nanoparticles: a comparison of different methods and algorithms. J Phys: Condens Matter 21:084208
Rossi G, Ferrando R (2017) Combining shape-changing with exchange moves in the optimization of nanoalloys. Comput Theore Chem 1107:66–73
Rossi G, Rapallo A, Mottet C, Fortunelli A, Baletto F, Ferrando R (2004) Magic polyicosahedral core-shell clusters. Phys Rev Lett 93(10):105503
Rossi K, Pavan L, Soon YY, Baletto F (2018) The effect of size and composition on structural transitions in monometallic nanoparticles. Eur Phys J B 91:33
Rzyman K, Moser Z (2004) Calorimetric studies of the enthalpies of formation of Al3Ni2, AlNi and AlNi3. Prog Mater Sci 49:581–606
Souza DGD, Cezar HM, Rondina GG, de. Oliveira MF, Silva JLFD (2016) A basin-hopping Monte Carlo investigation of the structural and energetic properties of 55- and 561-atom bimetallic nanoclusters: the examples of the ZrCu, ZrAl, and CuAl systems. J Phys: Condens Matter 28 (15):175302
Sundaram DS, Puri P, Yang V (2013) Thermochemical behavior of Ni-coated nanoalluminum particles. J Phys Chem C 117:7858–7869
Tyson WR, Miller WA (1977) Surface free energies of solid metals: estimation from liquid surface tension measurements. Surf Sci 62:267–276
Villars P, Calvert LD (1986) Pearson’s handbook of crystallographic data for intermetallic phases. American Society for Metals, Metals Park
Vitos L, Ruban AV, Skriver HL, Kollár J (1998) The surface energy of metals. Surf Sci 411:186–202
Wang Y, Liu ZK, Chen LQ (2004) Thermodynamic properties of Al, Ni, NiAl, and Ni3Al from first-principles calculations. Acta Mater 53:2665–2671
Wang GF, Hove MAV, Ross PN, Baskes MI (2005) Quantitative prediction of surface segregation in bimetallic Pt-M alloy nanoparticles (M = Ni, Re, Mo). Prog Surf Sci 79:28–45
Wen Y, Zhang J (2007) Surface energy calculation of the fcc metals by using the MAEAM. Solid State Commu 144:163–167
Yang J, Hu W, Xiao S, Yi G (2007) Surface melting of close-packed Mg(0001), compared with Al(111). Phys Sta Sol (B) 244:1913–1924
Yang J, Hu W, Wu Y, Dai X (2012a) Diffusion and growth of nickel, iron and magnesium adatoms on the aluminum truncated octahedron: a molecular dynamics simulation. Surf Sci 606:971–980
Yang J, Hu W, Wu Y, Dai X (2012b) Substrate dependence of growth configurations for Co-Cu bimetallic clusters. Cryst Growth Des 12:2978–2985
Yang J, Hu W, Tang J, Dai X (2013) Temperature effects on growth configurations of Al atoms on an Fe rhombohedron, a molecular dynamics simulation. J nanopart Res 15:1719–1725
Yang J, Hu W, Tang J (2015) Influence of solid-liquid interface on the thermal stability of Li-Fe nanoalloy with rhombohedral structure, a molecular dynamics study. Thin Solid Films 593:137–143
Yang J, Hu W, Dai X (2018) Surface segregation and alloying of immiscible Li-Cu and miscible Li-Pb nanoalloys investigated by basin-hopping Monte Carlo method. Comput Mater Sci 154:371–379
Yun K, Cho Y, Cha P, Lee J, Nam H, Oh JS, Choi J, Lee S (2012) Monte Carlo simulation of the structure of Pt-based bimetallic nanoparticles. Acta Mater 60:4908–4916
Zhong Y, Yang M, Liu Z (2005) Contribution of first-principles energetics to Al-Mg thermodynamic modeling. CalPhad 29:303–311
Zhu B, Front A, Guesmi H, Creuze J, Legrand B, Mottet C (2017) Magic compositions in Pd-Au nanoalloys. Comput Theor Chem 1107:49–56
Funding
This work is financially supported by the National Natural Science Foundation of China (Nos. 51271075, 51701071), by Scientific Research Fund of Hunan Provincial Education Department(No.15C0324).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J., Zhang, Y., Liu, Y. et al. A comparative atomic simulation study of the configurations in M-Al (M = Mg, Ni, and Fe) nanoalloys: influence of alloying ability, surface energy, atomic radius, and atomic arrangement. J Nanopart Res 22, 61 (2020). https://doi.org/10.1007/s11051-020-4756-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-020-4756-2