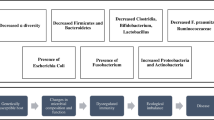
Abstract
Reduction in diversity of the intestinal microbiome (dysbiosis) is being identified in many disease states, and studies are showing important biologic contributions of microbiome to health and disease. Fecal microbiota transplantation (FMT) is being evaluated as a way to reverse dysbiosis in diseases and disorders in an attempt to improve health. The published literature was reviewed to determine the value of FMT in the treatment of medical disorders for which clinical trials have recently been conducted. FMT is effective in treating recurrent C. difficile infection in one or two doses, with many healthy donors providing efficacious fecal-derived products. In inflammatory bowel disease (IBD), FMT may lead to remission in approximately one-third of moderate-to-severe illnesses with one study suggesting that more durable FMT responses may be seen when used once medical remissions have been achieved. Donor products differ in their efficacy in treatment of IBD. Combining donor products has been one way to increase the potential value of FMT in treating chronic disorders. FMT is being explored in a variety of clinical settings affecting different organ systems outside CDI, with positive preliminary signals, in treatment of functional constipation, immunotherapy-induced colitis, neurodegenerative disease, as well as prevention of cancer-related disorders like graft versus host disease and decolonization of patients with recurrent urinary tract infection due to antibiotic-resistant bacteria. Currently, intense research is underway to see how the microbiome products like FMT can be harnessed for health benefits.

Similar content being viewed by others
References
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810.
Li J, Jia H, Cai X, et al. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32:834–841.
Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854–859.
Al-Jashaami LS, DuPont HL. Management of clostridium difficile infection. Gastroenterol Hepatol (N Y). 2016;12:609–616.
Human Microbiome Project C. Structure function and diversity of the healthy human microbiome. Nature. 2012;486:207–214.
Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: a common factor in human diseases. Biomed Res Int. 2017;2017:9351507.
Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503.
Zuo T, Wong SH, Cheung CP, et al. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat Commun. 2018;9:3663.
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191.
Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–1049.
Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The super-donor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol. 2019;9:2.
McSweeney B, Allegretti JR, Fischer M, et al. In search of stool donors: a multicenter study of prior knowledge, perceptions, motivators, and deterrents among potential donors for fecal microbiota transplantation. Gut Microbes. 2019;11:51–62.
Fang H, Fu L, Wang J. Protocol for fecal microbiota transplantation in inflammatory bowel disease: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:8941340.
Papanicolas LE, Choo JM, Wang Y, et al. Bacterial viability in faecal transplants: Which bacteria survive? EBioMedicine. 2019;41:509–516.
Chu ND, Smith MB, Perrotta AR, Kassam Z, Alm EJ. Profiling living bacteria informs preparation of fecal microbiota transplantations. PLoS ONE. 2017;12:e0170922.
Jiang ZD, Jenq RR, Ajami NJ, et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: a randomized clinical trial. PLoS ONE. 2018;13:e0205064.
Jiang ZD, Ajami NJ, Petrosino JF, et al. Randomised clinical trial: faecal microbiota transplantation for recurrent Clostridum difficile infection—fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther. 2017;45:899–908.
Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent clostridium difficile infection: a randomized clinical trial. JAMA. 2016;315:142–149.
Tang G, Yin W, Liu W. Is frozen fecal microbiota transplantation as effective as fresh fecal microbiota transplantation in patients with recurrent or refractory Clostridium difficile infection: a meta-analysis? Diagn Microbiol Infect Dis. 2017;88:322–329.
Costello SP, Conlon MA, Vuaran MS, Roberts-Thomson IC, Andrews JM. Faecal microbiota transplant for recurrent Clostridium difficile infection using long-term frozen stool is effective: clinical efficacy and bacterial viability data. Aliment Pharmacol Ther. 2015;42:1011–1018.
Bircher L, Geirnaert A, Hammes F, Lacroix C, Schwab C. Effect of cryopreservation and lyophilization on viability and growth of strict anaerobic human gut microbes. Microb Biotechnol. 2018;11:721–733.
Staley C, Hamilton MJ, Vaughn BP, et al. Successful resolution of recurrent clostridium difficile infection using freeze-dried, encapsulated fecal microbiota; pragmatic cohort study. Am J Gastroenterol. 2017;112:940–947.
Anand R, Song Y, Garg S, et al. Effect of aging on the composition of fecal microbiota in donors for FMT and its impact on clinical outcomes. Dig Dis Sci. 2017;62:1002–1008.
Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318:1985–1993.
Allegretti JR, Fischer M, Sagi SV, et al. Fecal microbiota transplantation capsules with targeted colonic versus gastric delivery in recurrent clostridium difficile infection: a comparative cohort analysis of high and lose dose. Dig Dis Sci. 2019;64:1672–1678.
Ossorio PN, Zhou Y. Regulating stool for microbiota transplantation. Gut Microbes. 2019;10:105–108.
Verbeke F, Janssens Y, Wynendaele E, De Spiegeleer B. Faecal microbiota transplantation: a regulatory hurdle? BMC Gastroenterol. 2017;17:128.
Olle B. Medicines from microbiota. Nat Biotechnol. 2013;31:309–315.
Kump P, Wurm P, Grochenig HP, et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment Pharmacol Ther. 2018;47:67–77.
Ma Y, Liu J, Rhodes C, Nie Y, Zhang F. Ethical issues in fecal microbiota transplantation in practice. Am J Bioeth. 2017;17:34–45.
Khanna S, Shin A, Kelly CP. Management of clostridium difficile infection in inflammatory bowel disease: expert review from the clinical practice updates committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15:166–174.
Meighani A, Hart BR, Bourgi K, Miller N, John A, Ramesh M. Outcomes of fecal microbiota transplantation for clostridium difficile infection in patients with inflammatory bowel disease. Dig Dis Sci. 2017;62:2870–2875.
Cheng YW, Phelps E, Ganapini V, et al. Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: a multicenter experience. Am J Transplant. 2019;19:501–511.
Hefazi M, Patnaik MM, Hogan WJ, Litzow MR, Pardi DS, Khanna S. Safety and efficacy of fecal microbiota transplant for recurrent clostridium difficile infection in patients with cancer treated with cytotoxic chemotherapy: a single-institution retrospective case series. Mayo Clin Proc. 2017;92:1617–1624.
Mintz M, Khair S, Grewal S, et al. Longitudinal microbiome analysis of single donor fecal microbiota transplantation in patients with recurrent Clostridium difficile infection and/or ulcerative colitis. PLoS ONE. 2018;13:e0190997.
Shogbesan O, Poudel DR, Victor S, et al. A systematic review of the efficacy and safety of fecal microbiota transplant for clostridium difficile infection in immunocompromised patients. Can J Gastroenterol Hepatol. 2018;2018:1394379.
Moss EL, Falconer SB, Tkachenko E, et al. Long-term taxonomic and functional divergence from donor bacterial strains following fecal microbiota transplantation in immunocompromised patients. PLoS ONE. 2017;12:e0182585.
Tariq R, Pardi DS, Bartlett MG, Khanna S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis. 2019;68:1351–1358.
Khan MY, Dirweesh A, Khurshid T, Siddiqui WJ. Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:1309–1317.
Hvas CL, Dahl Jorgensen SM, Jorgensen SP, et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. Gastroenterology. 2019;156:1324–1332.
Hocquart M, Lagier JC, Cassir N, et al. Early Fecal Microbiota Transplantation Improves Survival in Severe Clostridium difficile Infections. Clin Infect Dis. 2018;66:645–650.
van Beurden YH, Nieuwdorp M, van de Berg P, Mulder CJJ, Goorhuis A. Current challenges in the treatment of severe Clostridium difficile infection: early treatment potential of fecal microbiota transplantation. Therap Adv Gastroenterol. 2017;10:373–381.
Juul FE, Garborg K, Bretthauer M, et al. Fecal microbiota transplantation for primary clostridium difficile infection. N Engl J Med. 2018;378:2535–2536.
Ianiro G, Valerio L, Masucci L, et al. Predictors of failure after single faecal microbiota transplantation in patients with recurrent Clostridium difficile infection: results from a 3-year, single-centre cohort study. Clin Microbiol Infect. 2017;23:337e1–e3.
Allegretti JR, Allegretti AS, Phelps E, Xu H, Kassam Z, Fischer M. Asymptomatic Clostridium difficile carriage rate post-fecal microbiota transplant is low: a prospective clinical and stool assessment. Clin Microbiol Infect. 2018;24:780e1–e3.
Staley C, Kaiser T, Vaughn BP, et al. Predicting recurrence of Clostridium difficile infection following encapsulated fecal microbiota transplantation. Microbiome. 2018;6:166.
Seekatz AM, Theriot CM, Rao K, et al. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe. 2018;53:64–73.
Hibbard J, Jiang ZD, DuPont HL. Fecal calprotectin and fecal indole predict outcome of fecal microbiota transplantation in subjects with recurrent Clostridium difficile infection. Anaerobe. 2019;56:102–105.
Farowski F, Solbach P, Tsakmaklis A, et al. Potential biomarkers to predict outcome of faecal microbiota transfer for recurrent Clostridioides difficile infection. Dig Liver Dis. 2019;51:944–951.
Jalanka J, Hillamaa A, Satokari R, Mattila E, Anttila VJ, Arkkila P. The long-term effects of faecal microbiota transplantation for gastrointestinal symptoms and general health in patients with recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2018;47:371–379.
Lee CH, Chai J, Hammond K, et al. Long-term durability and safety of fecal microbiota transplantation for recurrent or refractory Clostridioides difficile infection with or without antibiotic exposure. Eur J Clin Microbiol Infect Dis. 2019;38:1731–1735.
Mamo Y, Woodworth MH, Wang T, Dhere T, Kraft CS. Durability and long-term clinical outcomes of fecal microbiota transplant treatment in patients with recurrent clostridium difficile infection. Clin Infect Dis. 2018;66:1705–1711.
Allegretti JR, Kassam Z, Fischer M, Kelly C, Chan WW. Risk factors for gastrointestinal symptoms following successful eradication of clostridium difficile by fecal microbiota transplantation (FMT). J Clin Gastroenterol. 2019;53:e405–e408.
Sethi S, Garey KW, Arora V, et al. Increased rate of irritable bowel syndrome and functional gastrointestinal disorders after Clostridium difficile infection. J Hosp Infect. 2011;77:172–173.
Smillie CS, Sauk J, Gevers D, et al. Strain tracking reveals the determinants of bacterial engraftment in the human gut following fecal microbiota transplantation. Cell Host Microbe. 2018;23:229–240.
Staley C, Kelly CR, Brandt LJ, Khoruts A, Sadowsky MJ. Complete microbiota engraftment is not essential for recovery from recurrent clostridium difficile infection following fecal microbiota transplantation. MBio. 2016;7;e01965-16.
Broecker F, Klumpp J, Moelling K. Long-term microbiota and virome in a Zurich patient after fecal transplantation against Clostridium difficile infection. Ann N Y Acad Sci. 2016;1372:29–41.
Brown JR, Flemer B, Joyce SA, et al. Changes in microbiota composition, bile and fatty acid metabolism, in successful faecal microbiota transplantation for Clostridioides difficile infection. BMC Gastroenterol. 2018;18:131.
Weingarden AR, Dosa PI, DeWinter E, et al. Changes in colonic bile acid composition following fecal microbiota transplantation are sufficient to control clostridium difficile germination and growth. PLoS ONE. 2016;11:e0147210.
Cheng S, Ma X, Geng S, et al. Fecal microbiota transplantation beneficially regulates intestinal mucosal autophagy and alleviates gut barrier injury. MSystems. 2018;3:e00137-18.
Ott SJ, Waetzig GH, Rehman A, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology. 2017;152:799–811.
Draper LA, Ryan FJ, Smith MK, et al. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome. 2018;6:220.
Zuo T, Wong SH, Lam K, et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut. 2018;67:634–643.
Lusiak-Szelachowska M, Weber-Dabrowska B, Jonczyk-Matysiak E, Wojciechowska R, Gorski A. Bacteriophages in the gastrointestinal tract and their implications. Gut Pathog. 2017;9:44.
DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol. 2011;8:523–531.
Cold F, Browne PD, Gunther S, et al. Multidonor FMT capsules improve symptoms and decrease fecal calprotectin in ulcerative colitis patients while treated—an open-label pilot study. Scand J Gastroenterol. 2019;54:289–296.
Mizuno S, Nanki K, Matsuoka K, et al. Single fecal microbiota transplantation failed to change intestinal microbiota and had limited effectiveness against ulcerative colitis in Japanese patients. Intest Res. 2017;15:68–74.
Conceicao-Neto N, Deboutte W, Dierckx T, et al. Low eukaryotic viral richness is associated with faecal microbiota transplantation success in patients with UC. Gut. 2018;67:1558–1559.
Gogokhia L, Buhrke K, Bell R, et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe. 2019;25:285–299.
Ding X, Li Q, Li P, et al. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf. 2019;42:869–880.
Sood A, Mahajan R, Singh A, et al. Role of fecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohns Colitis. 2019;13:1311–1317.
Herfarth H, Barnes EL, Long MD, et al. Combined endoscopic and oral fecal microbiota transplantation in patients with antibiotic-dependent pouchitis: low clinical efficacy due to low donor microbial engraftment. Inflamm Intest Dis. 2019;4:1–6.
De Palma G, Lynch MD, Lu J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9:eaaf6397.
Halkjaer SI, Christensen AH, Lo BZS, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018;67:2107–2115.
Huang HL, Chen HT, Luo QL, et al. Relief of irritable bowel syndrome by fecal microbiota transplantation is associated with changes in diversity and composition of the gut microbiota. J Dig Dis. 2019;20:401–408.
Johnsen PH, Hilpusch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3:17–24.
Mazzawi T, Lied GA, Sangnes DA, et al. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS ONE. 2018;13:e0194904.
Xu D, Chen VL, Steiner CA, et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114:1043–1050.
Ge X, Tian H, Ding C, et al. Fecal microbiota transplantation in combination with soluble dietary fiber for treatment of slow transit constipation: a pilot study. Arch Med Res. 2016;47:236–242.
Tian H, Ge X, Nie Y, et al. Fecal microbiota transplantation in patients with slow-transit constipation: a randomized, clinical trial. PLoS ONE. 2017;12:e0171308.
Gu L, Ding C, Tian H, et al. Serial frozen fecal microbiota transplantation in the treatment of chronic intestinal pseudo-obstruction: a preliminary study. J Neurogastroenterol Motil. 2017;23:289–297.
Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214.
Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2:ofv004.
Fischer M, Kao D, Kassam Z, et al. Stool donor body mass index does not affect recipient weight after a single fecal microbiota transplantation for clostridium difficile infection. Clin Gastroenterol Hepatol. 2018;16:1351–1353.
Cai TT, Ye XL, Yong HJ, et al. Fecal microbiota transplantation relieve painful diabetic neuropathy: a case report. Medicine (Baltimore). 2018;97:e13543.
Wieland A, Frank DN, Harnke B, Bambha K. Systematic review: microbial dysbiosis and nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2015;42:1051–1063.
Fitriakusumah Y, Lesmana CRA, Bastian WP, et al. The role of small intestinal bacterial overgrowth (SIBO) in non-alcoholic fatty liver disease (NAFLD) patients evaluated using controlled attenuation parameter (CAP) transient elastography (TE): a tertiary referral center experience. BMC Gastroenterol. 2019;19:43.
Rabot S, Membrez M, Bruneau A, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948–4959.
Bajaj JS, Kakiyama G, Savidge T, et al. Antibiotic-associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology. 2018;68:1549–1558.
Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017;66:1727–1738.
Mehta R, Kabrawala M, Nandwani S, et al. Preliminary experience with single fecal microbiota transplant for treatment of recurrent overt hepatic encephalopathy—a case series. Indian J Gastroenterol. 2018;37:559–562.
Philips CA, Phadke N, Ganesan K, Augustine P. Healthy donor faecal transplant for corticosteroid non-responsive severe alcoholic hepatitis. BMJ Case Rep. 2017;2017:bcr-2017.
Biagi E, Zama D, Rampelli S, et al. Early gut microbiota signature of aGvHD in children given allogeneic hematopoietic cell transplantation for hematological disorders. BMC Med Genom. 2019;12:49.
DeFilipp Z, Peled JU, Li S, et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018;2:745–753.
Taur Y, Coyte K, Schluter J, et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med. 2018;10:eaap9489.
Qi X, Li X, Zhao Y, et al. Treating steroid refractory intestinal acute graft-vs.-host disease with fecal microbiota transplantation: a pilot study. Front Immunol. 2018;9:2195.
Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24:1804–1808.
Gu B, Bo GZ, Ke C. Exploration of fecal microbiota transplantation in the treatment of refractory diarrhea after renal transplantation. Transplant Proc. 2018;50:1326–1331.
Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, Waubant E. The gut microbiome in human neurological disease: a review. Ann Neurol. 2017;81:369–382.
Kim S, Kwon SH, Kam TI, et al. Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson’s disease. Neuron. 2019;103:627–641.
Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480.
Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364:eaau6323.
Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358.
Davido B, Batista R, Michelon H, et al. Is faecal microbiota transplantation an option to eradicate highly drug-resistant enteric bacteria carriage? J Hosp Infect. 2017;95:433–437.
Dinh A, Fessi H, Duran C, et al. Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: a prospective comparative study. J Hosp Infect. 2018;99:481–486.
Singh R, de Groot PF, Geerlings SE, et al. Fecal microbiota transplantation against intestinal colonization by extended spectrum beta-lactamase producing Enterobacteriaceae: a proof of principle study. BMC Res Notes. 2018;11:190.
Sohn KM, Cheon S, Kim YS. Can fecal microbiota transplantation (FMT) eradicate fecal colonization with vancomycin-resistant enterococci (VRE)? Infect Control Hosp Epidemiol. 2016;37:1519–1521.
Leung V, Vincent C, Edens TJ, Miller M, Manges AR. Antimicrobial resistance gene acquisition and depletion following fecal microbiota transplantation for recurrent clostridium difficile infection. Clin Infect Dis. 2018;66:456–457.
Millan B, Park H, Hotte N, et al. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent clostridium difficile infection. Clin Infect Dis. 2016;62:1479–1486.
Stalenhoef JE, Terveer EM, Knetsch CW, et al. Fecal microbiota transfer for multidrug-resistant gram-negatives: a clinical success combined with microbiological failure. Open Forum Infect Dis. 2017;4:ofx047.
Battipaglia G, Malard F, Rubio MT, et al. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematological malignancies carrying multidrug-resistance bacteria. Haematologica. 2019;104:1682–1688.
Allegretti JR, Kassam Z, Carrellas M, et al. Fecal microbiota transplantation in patients with primary sclerosing cholangitis: a pilot clinical trial. Am J Gastroenterol. 2019;114:1071–1079.
Gunaltay S, Rademacher L, Hultgren Hornquist E, Bohr J. Clinical and immunologic effects of faecal microbiota transplantation in a patient with collagenous colitis. World J Gastroenterol. 2017;23:1319–1324.
van Beurden YH, van Gils T, van Gils NA, Kassam Z, Mulder CJ, Aparicio-Pages N. Serendipity in refractory celiac disease: full recovery of duodenal villi and clinical symptoms after fecal microbiota transfer. J Gastrointestin Liver Dis. 2016;25:385–388.
Rhoades N, Mendoza N, Jankeel A, et al. Altered immunity and microbial dysbiosis in aged individuals with long-term controlled HIV infection. Front Immunol. 2019;10:463.
Vujkovic-Cvijin I, Rutishauser RL, Pao M, et al. Limited engraftment of donor microbiome via one-time fecal microbial transplantation in treated HIV-infected individuals. Gut Microbes. 2017;8:440–450.
Rebello D, Wang E, Yen E, Lio PA, Kelly CR. Hair growth in two alopecia patients after fecal microbiota transplant. ACG Case Rep J. 2017;4:e107.
Li Q, Han Y, Dy ABC, Hagerman RJ. The gut microbiota and autism spectrum disorders. Front Cell Neurosci. 2017;11:120.
Kang Y, Cai Y. Future prospect of faecal microbiota transplantation as a potential therapy in asthma. Allergol Immunopathol (Madr). 2018;46:307–309.
Kragsnaes MS, Kjeldsen J, Horn HC, et al. Efficacy and safety of faecal microbiota transplantation in patients with psoriatic arthritis: protocol for a 6-month, double-blind, randomised, placebo-controlled trial. BMJ Open. 2018;8:e019231.
Kelly CR, Kim AM, Laine L, Wu GD. The AGA’s fecal microbiota transplantation national registry: an important step toward understanding risks and benefits of microbiota therapeutics. Gastroenterology. 2017;152:681–684.
Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol. 2013;108:1367.
Brooks PT, Brakel KA, Bell JA, et al. Transplanted human fecal microbiota enhanced Guillain Barre syndrome autoantibody responses after Campylobacter jejuni infection in C57BL/6 mice. Microbiome. 2017;5:92.
Fischer M, Bittar M, Papa E, Kassam Z, Smith M. Can you cause inflammatory bowel disease with fecal transplantation? A 31-patient case-series of fecal transplantation using stool from a donor who later developed Crohn’s disease. Gut Microbes. 2017;8:205–207.
Qazi T, Amaratunga T, Barnes EL, Fischer M, Kassam Z, Allegretti JR. The risk of inflammatory bowel disease flares after fecal microbiota transplantation: Systematic review and meta-analysis. Gut Microbes. 2017;8:574–588.
Tran V, Phan J, Nulsen B, et al. Severe ileocolonic Crohn’s disease flare associated with fecal microbiota transplantation requiring diverting ileostomy. ACG Case Rep J. 2018;5:e97.
Jakobsson HE, Rodriguez-Pineiro AM, Schutte A, et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16:164–177.
Yu LC, Wang JT, Wei SC, Ni YH. Host-microbial interactions and regulation of intestinal epithelial barrier function: from physiology to pathology. World J Gastrointest Pathophysiol. 2012;3:27–43.
Fuentes S, Rossen NG, van der Spek MJ, et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017;11:1877–1889.
Paramsothy S, Nielsen S, Kamm MA, et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology. 2019;156:1440–1454.
Mazzawi T, Hausken T, Hov JR, et al. Clinical response to fecal microbiota transplantation in patients with diarrhea-predominant irritable bowel syndrome is associated with normalization of fecal microbiota composition and short-chain fatty acid levels. Scand J Gastroenterol. 2019;54:690–699.
Tian Z, Liu J, Liao M, et al. Beneficial effects of fecal microbiota transplantation on ulcerative colitis in mice. Dig Dis Sci. 2016;61:2262–2271.
Burrello C, Garavaglia F, Cribiu FM, et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat Commun. 2018;9:5184.
Kakihana K, Fujioka Y, Suda W, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128:2083–2088.
Jacob V, Crawford C, Cohen-Mekelburg S, et al. Single delivery of high-diversity fecal microbiota preparation by colonoscopy is safe and effective in increasing microbial diversity in active ulcerative colitis. Inflamm Bowel Dis. 2017;23:903–911.
Vaughn BP, Vatanen T, Allegretti JR, et al. Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. Inflamm Bowel Dis. 2016;22:2182–2190.
Kim M, Galan C, Hill AA, et al. Critical role for the microbiota in CX3CR1(+) intestinal mononuclear phagocyte regulation of intestinal T cell responses. Immunity. 2018;49:151–163.
Okai S, Usui F, Yokota S, et al. High-affinity monoclonal IgA regulates gut microbiota and prevents colitis in mice. Nat Microbiol. 2016;1:16103.
Okai S, Usui F, Ohta M, et al. Intestinal IgA as a modulator of the gut microbiota. Gut Microbes. 2017;8:486–492.
Cheng CS, Wei HK, Wang P, et al. Early intervention with faecal microbiota transplantation: an effective means to improve growth performance and the intestinal development of suckling piglets. Animal. 2019;13:533–541.
Kazerouni A, Wein LM. Exploring the efficacy of pooled stools in fecal microbiota transplantation for microbiota-associated chronic diseases. PLoS ONE. 2017;12:e0163956.
Olesen SW, Gurry T, Alm EJ. Designing fecal microbiota transplant trials that account for differences in donor stool efficacy. Stat Methods Med Res. 2018;27:2906–2917.
Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109.
Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218–1228.
Costello SP, Hughes PA, Waters O, et al. Effect of Fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321:156–164.
Cao Y, Zhang B, Wu Y, Wang Q, Wang J, Shen F. The value of fecal microbiota transplantation in the treatment of ulcerative colitis patients: a systematic review and meta-analysis. Gastroenterol Res Pract. 2018;2018:5480961.
Ishikawa D, Sasaki T, Takahashi M, et al. The microbial composition of bacteroidetes species in ulcerative colitis is effectively improved by combination therapy with fecal microbiota transplantation and antibiotics. Inflamm Bowel Dis. 2018;24:2590–2598.
Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149:110–118.
Imdad A, Nicholson MR, Tanner-Smith EE, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2018;11:CD012774.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HLD and Z-DJ have applied for a patent for their FMT product and HLD has received a grant from Rebiotix to study their FMT product.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
DuPont, H.L., Jiang, ZD., DuPont, A.W. et al. Abnormal Intestinal Microbiome in Medical Disorders and Potential Reversibility by Fecal Microbiota Transplantation. Dig Dis Sci 65, 741–756 (2020). https://doi.org/10.1007/s10620-020-06102-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06102-y