Abstract
Background
Acute pancreatitis (AP) is a sudden inflammation of the pancreas that may be life-threatening disease with high mortality rates, particularly in the presence of systemic inflammatory response and multiple organ failure. Oxidative stress has been shown to be involved in the pathophysiology of acute pancreatitis.
Aim
This study is designed to investigate the possible effect of mesna on an experimental model of cerulein-induced acute pancreatitis.
Methods
Animals were divided into five groups: Group 1 served as a control group given the saline; group II (mesna group) received mesna at a dose of (100 mg/kg per dose, i.p.) four times; group III (acute pancreatitis group) received cerulein at a dose of (20 µg/kg/dose, s.c.) four times with 1-h intervals; group VI, cerulein + mesna, was treated with mesna at a dose of (100 mg/kg, i.p.) 15 min before each cerulein injection.
Results
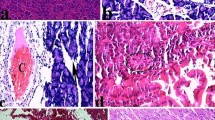
Animals with acute pancreatitis showed elevated serum amylase and lipase levels. Biochemical parameters showed increased pancreatic tumor necrosis factors-α (TNF-α) and interleukin-1β (IL-1β) levels. A disturbance in oxidative stress markers was evident by elevated pancreatic lipid peroxides (TBARS) and decline in pancreatic antioxidants’ concentrations including reduced glutathione (GSH); superoxide dismutase (SOD); and glutathione peroxidase (GSH-Px). Histological examination confirmed pancreatic injury. Pre-treatment with mesna was able to abolish the changes in pancreatic enzymes, oxidative stress markers (TBARS, SOD, GSH and GSH-Px), pancreatic inflammatory markers (TNF-α, IL-1β) as well as histological changes.
Conclusions
Mesna mitigates AP by alleviating pancreatic oxidative stress damage and inhibiting inflammation.




Similar content being viewed by others
References
Conti Bellocchi MC, Campagnola P, Frulloni L. Drug-induced acute pancreatitis. Pancreapedia Exocrine Pancreas Knowl Base. 2015;1:5. https://doi.org/10.3998/panc.2015.32.
Almeida JL, Sampietre SN, Mendonça Coelho AM, et al. Statin pretreatment in experimental acute pancreatitis. JOP. 2008;9(4):431–439.
Portelli M, Jones CD. Severe acute pancreatitis: pathogenesis, diagnosis and surgical management. Hepatobiliary Pancreat Dis Int. 2017;16(2):155–159.
Majidi S, Golembioski A, Wilson SL, Thompson EC. Acute pancreatitis: etiology, pathology, diagnosis, and treatment. South Med J. 2017;110(11):727–732.
Adiamah AE, Crook PM, Lobo DN. A systematic review of the epidemiology, pathophysiology and current management of hyperlipidaemic pancreatitis. Clin Nutr. 2018;37(6 Pt A):1810–1822.
Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375(20):1972–1981.
Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal. 2009;11(1):135–165.
Dios ID. Inflammatory role of the acinar cells during acute pancreatitis. World J Gastrointest Pharmacol Ther. 2010;1(1):15–20.
Criddle DN. Reactive oxygen species, Ca (2+) stores and acute pancreatitis; a step closer to therapy? Cell Calcium. 2016;60(3):180–189.
Armstrong JA, Cash N, Soares PM, Souza MH, Sutton R, Criddle DN. Oxidative stress in acute pancreatitis: lost in translation? Free Radic Res. 2013;47(11):917–933.
Milnerowicz H, Bukowski R, Jablonowska M, Sciskalska M, Milnerowicz S. The antioxidant profiles, lysosomal and membrane enzymes activity in patients with acute pancreatitis. Mediators Inflamm. 2014;2014:376518.
Abu-Zidan FM, Bonham MJ, Windsor JA. Severity of acute pancreatitis: a multivariate analysis of oxidative stress markers and modified Glasgow criteria. Br J Surg. 2000;87(8):1019–1023.
Norberg KJ, Nania S, Li X, et al. RCAN1 is a marker of oxidative stress, induced in acute pancreatitis. Pancreatology. 2018;18(7):734–741.
Solakoglu TH, Koseoglu S, Isikoglu O, Erel Ersoy O. Association between antioxidants and mild acute pancreatitis. Arab J Gastroenterol. 2017;18(4):201–205.
Kambhampati S, Park W, Habtezion A. Pharmacologic therapy for acute pancreatitis. World J Gastroenterol. 2014;20(45):16868–16880.
Esrefoglu M. Experimental and clinical evidence of antioxidant therapy in acute pancreatitis. World J Gastroenterol. 2012;18(39):5533–5541.
Carrasco C, Marchena AM, Holguin-Arevalo MS, et al. Anti-inflammatory effects of melatonin in a rat model of caerulein-induced acute pancreatitis. Cell Biochem Funct. 2013;31(7):585–590.
Ozkan E, Akyuz C, Dulundu E, et al. Protective effects of lycopene on cerulein-induced experimental acute pancreatitis in rats. J Surg Res. 2012;176(1):232–238.
Gressier B, Lebegue N, Brunet C, et al. Scavenging of reactive oxygen species by letosteine, a molecule with two blocked-SH groups Comparison with free-SH drugs. Pharm World Sci. 1995;17(3):76–80.
Skinner N, Sharkey IM, Pearson ADJ, Craft AW. Ifosfamide, mesna and nephrotoxicity in children. J Clin Oncol. 1993;11:173–190.
Triantafyllidis I, Poutahidis T, Taitzoglou I, Kesisoglou I, Lazaridis C, Botsios D. Treatment with Mesna and n-3 polyunsaturated fatty acids ameliorates experimental ulcerative colitis in rats. Int J Exp Pathol. 2015;96(6):433–443.
Ypsilantis P, Tentes I, Lambropoulou M, et al. Prophylaxis with mesna prevents oxidative stress induced by ischemia reperfusion in the intestine via inhibition of nuclear factor-kappa B activation. J Gastroenterol Hepatol. 2008;23(2):328–335.
Buyukberber M, Savas MC, Bagci C, et al. The beneficial effect of propolis on cerulein-induced experimental acute pancreatitis in rats. Turk J Gastroenterol. 2009;20(2):122–128.
Ypsilantis P, Lambropoulou M, Tentes I, Kortsaris A, Papadopoulos N, Simopoulos C. Mesna protects intestinal mucosa from ischemia/reperfusion injury. J Surg Res. 2006;134(2):278–284.
Buege JA, Aust SD. Microsomal lipid peroxidation methods. Enzymol. 1978;52:302–310.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77.
Nagi MN, Suneja SK, Cook L. Depletion of rat hepatic glutathione and inhibition of microsomal trans-2-Enoyl-CoA reductase activity following administration of Dec-2-ynol and Dec-2-ynoic acid. Arch Biochem Biophys. 1992;293:71–87.
Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–169.
Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497–500.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254.
Tani S, Otsuki M, Itoh H, et al. Histologic and biochemical alterations in experimental acute pancreatitis induced by supramaximal cerulein stimulation. Int J Pancreatol. 1987;2:337–348.
Dabrowski A, Gabryelewicz A, Wereszczynska-Siemiatkowska U, Chyczewski L. Oxygen-derived free radicals in cerulein-induced acute pancreatitis. Scand J Gastroenterol. 1988;23(10):1245–1249.
Goerelick FS, Adler G, Kem HF. Cerulein induced pancreatitis. In: Go VLW, DiMagno EP, Gardner JP, Lebenthal E, Reber HA, Scheele GA, eds. The pancreas: biology, pathobiology, and disease. 2nd ed. New York: Raven Press; 1993:501–526.
Matull WR, Pereira SP, O’Donohue JW. Biochemical markers of acute pancreatitis. J Clin Pathol.. 2006;59(4):340–344.
Chvanov M, Petersen OH, Tepikin A. Free radicals and the pancreatic acinar cells: role in physiology and pathology. Philos Trans R Soc Lond B Biol Sci.. 2005;360(1464):2273–2284.
Curran FJM, Sattar N, Talwar D, Baxter JN, Imrie CW. Relationship of carotenoid and vitamins A and E with the acute inflammatory response in acute pancreatitis. Br J Surg. 2000;87(3):301–305.
Amirshahrokhi K, Khalili AR. Gastroprotective effect of 2-mercaptoethane sulfonate against acute gastric mucosal damage induced by ethanol. Int Immunopharmacol. 2016;34:183–188.
Sener G, Sehirli O, Ercan F, Sirvanci S, Gedik N, Kacmaz A. Protective effect of MESNA (2-mercaptoethane sulfonate) against hepatic ischemia/reperfusion injury in rats. Surg Today. 2005;35(7):575–580.
Kabasakal L, Sehirli AO, Cetinel S, Cikler E, Gedik N, Sener G. Mesna (2-mercaptoethane sulfonate) prevents ischemia/reperfusion induced renal oxidative damage in rats. Life Sci. 2004;75(19):2329–2340.
Bopanna S, Nayak B, Prakash S, Shalimar, Mahapatra SJ, Garg PK. Increased oxidative stress and deficient antioxidant levels may be involved in the pathogenesis of idiopathic recurrent acute pancreatitis. Pancreatology. 2017;17(4):529–533.
Sener G, Sehirli O, Cetinel S, Yeğen BG, Gedik N, Ayanoğlu-Dülger G. Protective effects of MESNA (2-mercaptoethane sulphonate) against acetaminophen-induced hepatorenal oxidative damage in mice. J Appl Toxicol. 2005;25:20–29.
Sener G, Kabasakal L, Sehirli O, Ercan F, Gedik N. 2-Mercaptoethane sulfonate (MESNA) protects against biliary obstruction-induced oxidative damage in rats. Hepatol Res. 2006;35(2):140–146.
Al Maruf A, O’Brien PJ, Naserzadeh P, Fathian R, Salimi A, Pourahmad J. Methotrexate induced mitochondrial injury and cytochrome c release in rat liver hepatocytes. Drug Chem Toxicol. 2018;41(1):51–61.
Pereda J, Sabater L, Aparisi L, et al. Interaction between cytokines and oxidative stress in acute pancreatitis. Curr Med Chem. 2006;13(23):2775–2787.
Duan L, Ma Y, Chi J, Wang X, Wesley AJ, Chen X. The regulatory role of immunosuppressants on immune abnormalities in acute pancreatitis. Biomed Rep. 2014;2(2):193–198.
Watanabe T, Kudo M, Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol. 2017;10(2):283–298.
Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72(11):1493–1505.
Jeelani R, Jahanbakhsh S, Kohan-Ghadr HR, et al. Mesna (2-mercaptoethane sodium sulfonate) functions as a regulator of myeloperoxidase. Free Radic Biol Med. 2017;110:54–62.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the Undergraduate Research Support Program, Project no. (URSP-17-48).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hagar, H.H., Almubrik, S.A., Attia, N.M. et al. Mesna Alleviates Cerulein-Induced Acute Pancreatitis by Inhibiting the Inflammatory Response and Oxidative Stress in Experimental Rats. Dig Dis Sci 65, 3583–3591 (2020). https://doi.org/10.1007/s10620-020-06072-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06072-1