Abstract
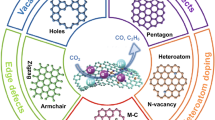
Developing efficient carbon-based metal-free electrocatalysts can bridge the gap between laboratory studies and practical applications of CO2 reduction. However, along with the ambiguous understanding of the active sites in carbon-based electrocatalysts, carbon-based electrocatalysts with high selectivity and satisfactory stability for electroreduction of CO2 remain rare. Here, using the nitrogen rich silk cocoon as a precursor, carbon-based electrocatalysts with intrinsic defects can be prepared for efficient and long-term electroreduction of CO2 by a simple two-step carbonization. The obtained electrocatalyst can catalyze CO2 reduction to CO with a Faradaic efficiency of ~ 89% and maintain good selectivity for about 10 days. Particularly, our experimental studies suggest that in-plane defects are the main active sites on which the rate-determining step for CO2 reduction should be the direct electron transfer to CO2 but not the proton-coupled electron transfer. Further theoretical calculations consistently demonstrate that the intrinsic defects in carbon matrix, particularly the pentagon-containing defects, act as main active sites to accelerate the direct electron transfer for CO2 reduction. In addition, our synthetic approach can convert egg white into efficient catalysts for CO2 electroreduction. These findings, providing new insights into the biomass-derived catalysts, should pave the way for fabricating efficient and stable carbon-based electrocatalysts with catalytically active defects by using naturally abundant precursors.

Similar content being viewed by others
References
Sun, T. T.; Xu, L. B.; Wang, D. S.; Li, Y. D. Metal organic frameworks derived single atom catalysts for electrocatalytic energy conversion. Nano Res.2019, 12, 2067–2080.
Song, R. B.; Zhu, W. L.; Fu, J. J.; Chen, Y.; Liu, L.; Zhang, J. R.; Lin, Y.; Zhu, J. J. Electrode materials engineering in electrocatalytic CO2 reduction: Energy input and conversion efficiency. Adv. Mater., in press, DOI: https://doi.org/10.1002/adma.201903796.
Wu, J. J.; Sharifi, T.; Gao, Y.; Zhang, T. Y.; Ajayan, P. M. Emerging carbon-based heterogeneous catalysts for electrochemical reduction of carbon dioxide into value-added chemicals. Adv. Mater.2019, 31, 1804257.
Zhu, D. D.; Liu, J. L.; Qiao, S. Z. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv. Mater.2016, 28, 3423–3452.
Lin, R.; Ma, X. L.; Cheong, W. C.; Zhang, C.; Zhu, W.; Pei, J. J.; Zhang, K. Y.; Wang, B.; Liang, S. Y.; Liu, Y. X. et al. PdAg bimetallic electrocatalyst for highly selective reduction of CO2 with low COOH* formation energy and facile CO desorption. Nano Res.2019, 12, 2866–2871.
Kumar, B.; Asadi, M.; Pisasale, D.; Sinha-Ray, S.; Rosen, B. A.; Haasch, R.; Abiade, J.; Yarin, A. L.; Salehi-Khojin, A. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction. Nat. Commun.2013, 4, 2819.
Liu, X.; Dai, L. M. Carbon-based metal-free catalysts. Nat. Rev. Mater.2016, 1, 16064.
Yan, X. C.; Jia, Y.; Yao, X. D. Defects on carbons for electrocatalytic oxygen reduction. Chem. Soc. Rev.2018, 47, 7628–7658.
Yan, X. C.; Jia, Y.; Odedairo, T.; Zhao, X. J.; Jin, Z.; Zhu, Z. H.; Yao, X. D. Activated carbon becomes active for oxygen reduction and hydrogen evolution reactions. Chem. Commun.2016, 52, 8156–8159.
Zhang, S.; Kang, P.; Ubnoske, S.; Brennaman, M. K.; Song, N.; House, R. L.; Glass, J. T.; Meyer, T. J. Polyethylenimine-enhanced electrocatalytic reduction of CO2 to formate at nitrogen-doped carbon nanomaterials. J. Am. Chem. Soc.2014, 136, 7845–7848.
Wang, H. X.; Chen, Y. B.; Hou, X. L.; Ma, C. Y.; Tan, T. W. Nitrogendoped graphenes as efficient electrocatalysts for the selective reduction of carbon dioxide to formate in aqueous solution. Green Chem.2016, 18, 3250–3256.
Kuang, M.; Guan, A. X.; Gu, Z. X.; Han, P.; Qian, L. P.; Zheng, G. F. Enhanced N-doping in mesoporous carbon for efficient electrocatalytic CO2 conversion. Nano Res.2019, 12, 2324–2329.
Wu, J. J.; Ma, S. C.; Sun, J.; Gold, J. I.; Tiwary, C. S.; Kim, B.; Zhu, L. Y.; Chopra, N.; Odeh, I. N.; Vajtai, R. et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates. Nat. Commun.2016, 7, 13869.
Cui, X. Q.; Pan, Z. Y.; Zhang, L. J.; Peng, H. S.; Zheng, G. F. Selective etching of nitrogen-doped carbon by steam for enhanced electrochemical CO2 reduction. Adv. Energy Mater.2017, 7, 1701456.
Ghausi, M. A.; Xie, J. F.; Li, Q. H.; Wang, X. Y.; Yang, R.; Wu, M.; Wang, Y.; Dai, L. CO2 overall splitting by a bifunctional metal-free electrocatalyst. Angew. Chem., Int. Ed.2018, 57, 13135–13139.
Chen, Z. P.; Mou, K. W.; Yao, S. Y.; Liu, L. C. Highly selective electrochemical reduction of CO2 to formate on metal-free nitrogendoped PC61BM. J. Mater. Chem. A2018, 6, 11236–11243.
Li, H. Q.; Xiao, N.; Hao, M. Y.; Song, X. D.; Wang, Y. W.; Ji, Y. Q.; Liu, C.; Li, C.; Guo, Z.; Zhang, F. et al. Efficient CO2 electroreduction over pyridinic-N active sites highly exposed on wrinkled porous carbon nanosheets. Chem. Eng. J.2018, 351, 613–621.
Tuci, G.; Filippi, J.; Ba, H.; Rossin, A.; Luconi, L.; Pham-Huu, C.; Vizza, F.; Giambastiani, G. How to teach an old dog new (electrochemical) tricks: Aziridine-functionalized CNTs as efficient electrocatalysts for the selective CO2 reduction to CO. J. Mater. Chem. A2018, 6, 16382–16389.
Hursán, D.; Samu, A. A.; Janovák, L.; Artyushkova, K.; Asset, T.; Atanassov, P.; Janáky, C. Morphological attributes govern carbon dioxide reduction on N-doped carbon electrodes. Joule2019, 3, 1719–1733.
Wang, W.; Shang, L.; Chang, G. J.; Yan, C. Y.; Shi, R.; Zhao, Y. X.; Waterhouse, G. I. N.; Yang, D. J.; Zhang, T. R. Intrinsic carbon-defectdriven electrocatalytic reduction of carbon dioxide. Adv. Mater.2019, 31, 1808276.
Jia, Y.; Zhang, L. Z.; Zhuang, L. Z.; Liu, H. L.; Yan, X. C.; Wang, X.; Liu, J. D.; Wang, J. C.; Zheng, Y. R.; Xiao, Z. H. et al. Identification of active sites for acidic oxygen reduction on carbon catalysts with and without nitrogen doping. Nat. Catal.2019, 2, 688–695.
Titirici, M. Defects win over pyridinic sites. Nat. Catal.2019, 2, 642–643.
Zhu, J. W.; Huang, Y. P.; Mei, W. C.; Zhao, C. Y.; Zhang, C. T.; Zhang, J.; Amiinu, I. S.; Mu, S. C. Effects of intrinsic pentagon defects on electrochemical reactivity of carbon nanomaterials. Angew. Chem., Int. Ed.2019, 58, 3859–3864.
Jiang, Y. F.; Yang, L. J.; Sun, T.; Zhao, J.; Lyu, Z. Y.; Zhuo, O.; Wang, X. Z.; Wu, Q.; Ma, J.; Hu, Z. Significant contribution of intrinsic carbon defects to oxygen reduction activity. ACS Catal.2015, 5, 6707–6712.
Daiyan, R.; Tan, X.; Chen, R.; Saputera, W. H.; Tahini, H. A.; Lovell, E.; Ng, Y. H.; Smith, S. C.; Dai, L. M.; Lu, X. Y. et al. Electroreduction of CO2 to CO on a mesoporous carbon catalyst with progressively removed nitrogen moieties. ACS Energy Lett.2018, 3, 2292–2298.
Li, W. L.; Herkt, B.; Seredych, M.; Bandosz, T. J. Pyridinic-N groups and ultramicropore nanoreactors enhance CO2 electrochemical reduction on porous carbon catalysts. Appl. Catal. B: Environ.2017, 207, 195–206.
Li, F. W.; Xue, M. Q.; Knowles, G. P.; Chen, L.; MacFarlane, D. R.; Zhang, J. Porous nitrogen-doped carbon derived from biomass for electrocatalytic reduction of CO2 to CO. Electrochim. Acta2017, 245, 561–568.
Lu, M.; Qian, Y. J.; Yang, C. C.; Huang, X.; Li, H.; Xie, X. J.; Huang, L.; Huang, W. Nitrogen-enriched pseudographitic anode derived from silk cocoon with tunable flexibility for microbial fuel cells. Nano Energy2017, 32, 382–388.
Cho, S. Y.; Yun, Y. S.; Lee, S.; Jang, D.; Park, K. Y.; Kim, J. K.; Kim, B. H.; Kang, K.; Kaplan, D. L.; Jin, H. J. Carbonization of a stable ß-sheet-rich silk protein into a pseudographitic pyroprotein. Nat. Commun.2015, 6, 7145.
Jones, F.; Tran, H.; Lindberg, D.; Zhao, L. M.; Hupa, M. Thermal stability of zinc compounds. Energy Fuels2013, 27, 5663–5669.
Caturla, F.; Molina-Sabio, M.; Rodríguez-Reinoso, F. Preparation of activated carbon by chemical activation with ZnCl2. Carbon1991, 29, 999–1007.
Liu, S.; Yang, H. B.; Huang, X.; Liu, L. H.; Cai, W. Z.; Gao, J. J.; Li, X. N.; Zhang, T.; Huang, Y. Q.; Liu, B. Identifying active sites of nitrogendoped carbon materials for the CO2 reduction reaction. Adv. Funct. Mater.2018, 28, 1800499.
Won, D. H.; Shin, H.; Koh, J.; Chung, J.; Lee, H. S.; Kim, H.; Woo, S. I. Highly efficient, selective, and stable CO2 electroreduction on a hexagonal Zn catalyst. Angew. Chem., Int. Ed.2016, 55, 9297–9300.
Yang, F.; Song, P.; Liu, X. Z.; Mei, B. B.; Xing, W.; Jiang, Z.; Gu, L.; Xu, W. L. Highly efficient CO2 electroreduction on ZnN4-based single-atom catalyst. Angew. Chem., Int. Ed.2018, 57, 12303–12307.
Wu, Y. S.; Jiang, J. B.; Weng, Z.; Wang, M. Y.; Broere, D. L. J.; Zhong, Y. R.; Brudvig, G. W.; Feng, Z. X.; Wang, H. L. Electroreduction of CO2 catalyzed by a heterogenized Zn-porphyrin complex with a redox-innocent metal center. ACS Cent. Sci.2017, 3, 847–852.
Song, Y. F.; Chen, W.; Zhao, C. C.; Li, S. G.; Wei, W.; Sun, Y. H. Metal-free nitrogen-doped mesoporous carbon for electroreduction of CO2 to ethanol. Angew. Chem., Int. Ed.2017, 56, 10840–10844.
Wang, R. M.; Sun, X. H.; Ould-Chikh, S.; Osadchii, D.; Bai, F.; Kapteijn, F.; Gascon, J. Metal-organic-framework-mediated nitrogendoped carbon for CO2 electrochemical reduction. ACS Appl. Mater. Interfaces2018, 10, 14751–14758.
Wang, H.; Jia, J.; Song, P. F.; Wang, Q.; Li, D. B.; Min, S. X.; Qian, C. X.; Wang, L.; Li, Y. F.; Ma, C. et al. Efficient electrocatalytic reduction of CO2 by nitrogen-doped nanoporous carbon/carbon nanotube membranes: A step towards the electrochemical CO2 refinery. Angew. Chem., Int. Ed.2017, 56, 7847–7852.
Lee, C. W.; Cho, N. H.; Im, S. W.; Jee, M. S.; Hwang, Y. J.; Min, B. K.; Nam, K. T. New challenges of electrokinetic studies in investigating the reaction mechanism of electrochemical CO2 reduction. J. Mater. Chem. A2018, 6, 14043–14057.
Medina-Ramos, J.; DiMeglio, J. L.; Rosenthal, J. Efficient reduction of CO2 to CO with high current density using in situ or ex situ prepared bi-based materials. J. Am. Chem. Soc.2014, 136, 8361- 8367.
Wuttig, A.; Yoon, Y.; Ryu, J.; Surendranath, Y. Bicarbonate is not a general acid in Au-catalyzed CO2 electroreduction. J. Am. Chem. Soc.2017, 139, 17109–17113.
Acknowledgements
The authors thank the National Key R&D Program of China (No. 2017YFA0207201), Six Talent Peaks Project in Jiangsu Province (No. JNHB-038), and Young Elite Scientists Sponsorship Program by CAST (No. 2017QNRC001) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Chen, M., Wang, S., Zhang, H. et al. Intrinsic defects in biomass-derived carbons facilitate electroreduction of CO2. Nano Res. 13, 729–735 (2020). https://doi.org/10.1007/s12274-020-2683-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2683-2