Abstract
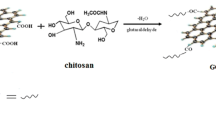
In this study, a graphene oxide nanoribbons/chitosan (GONRs/CTS) composite membrane was successfully prepared by encapsulating CTS into GONRs, which were unzipped from multi-walled carbon nanotubes. The GONRs/CTS composite membrane so prepared was characterized using scanning electron microscopy, X-Ray diffraction and Fourier transform infrared spectroscopy. The effects of the experimental conditions such as the pH (2‒7), adsorbent dosage (10‒50 mg), experimental time (5 min–32 h), uranium concentration (25‒300 mg∙L–1), experimental temperature (298 K‒328 K) on the adsorption properties of the composite membrane for the removal of U(VI) were investigated. The results showed that the U(VI) adsorption process of the GONRs/CTS composite membrane was pH-dependent, rapid, spontaneous and endothermic. The adsorption process followed the pseudosecondary kinetics and Langmuir models. The maximum U(VI) adsorption capacity of the GONRs/CTS composite membrane was calculated to be 320 mg∙g−1. Hence, the GONRs/CTS composite membrane prepared in this study was found to be suitable for separating and recovering uranium from wastewater.

Similar content being viewed by others
References
Selvakumar R, Ramadoss G, Menon M, Rajendran K, Thavamani P, Naidu R, Megharaj M. Challenges and complexities in remediation of uranium contaminated soils: A review. Journal of Environmental Radioactivity, 2018, 192: 592–603
Lesher E, Ranville J, Honeyman B. Analysis of pH dependent uranium(VI) sorption to nanoparticulate hematite by flow field-elow fractionation-inductively coupled plasma mass spectrometry. Environmental Science & Technology, 2009, 43(14): 5403–5409
Zhu J, Liu Q, Li Z, Liu J, Zhang H, Li R, Wang J. Efficient extraction of uranium from aqueous solution using an aminofunctionalized magnetic titanate nanotubes. Journal of Hazardous Materials, 2018, 353: 9–17
Amphlett J, Ogden M, Foster R, Syna N, Soldenhoff K, Sharrad C. The effect of contaminants on the application of polyamine functionalised ion exchange resins for uranium extraction from sulfate based mining process waters. Chemical Engineering Journal, 2018, 354: 633–640
Bertoli A, Quintão M, De Abreu H, Ladeira A, Duarte H. Uranium separation from acid mine drainage using anionic resins—An experimental/theoretical investigation of its chemical speciation and the interaction mechanism. Journal of Environmental Chemical Engineering, 2019, 7(1): 102790
Cheng Y, He P, Dong F, Nie X, Ding C, Wang S, Zhang Y, Liu H, Zhou S. Polyamine and amidoxime groups modified bifunctional polyacrylonitrile-based ion exchange fibers for highly efficient extraction of U(VI) from real uranium mine water. Chemical Engineering Journal, 2019, 367: 198–207
Torkabad M, Keshtkar A, Safdari S. Comparison of polyethersulfone and polyamide nanofiltration membranes for uranium removal from aqueous solution. Progress in Nuclear Energy, 2017, 94: 93–100
Lakaniemi A, Douglas G, Kaksonen A. Engineering and kinetic aspects of bacterial uranium reduction for the remediation of uranium contaminated environments. Journal of Hazardous Materials, 2019, 371: 198–212
Nariyan E, Sillanpää M, Wolkersdorfer C. Uranium removal from pyhäsalmi/finland mine water by batch electrocoagulation and optimization with the response surface methodology. Separation and Purification Technology, 2018, 193: 386–397
Wu Y, Chen D, Kong L, Tsang D, Su M. Rapid and effective removal of uranium(VI) from aqueous solution by facile synthesized hierarchical hollow hydroxyapatite microspheres. Journal of Hazardous Materials, 2019, 371: 397–405
Pan N, Li L, Ding J, Li S, Wang R, Jin Y, Wang X, Xia C. Preparation of graphene oxide-manganese dioxide for highly efficient adsorption and separation of Th(IV)/U(VI). Journal of Hazardous Materials, 2016, 309: 107–115
Sun M, Li J. Graphene oxide membranes: Functional structures, preparation and environmental applications. Nano Today, 2018, 20: 121–137
Tan L, Liu Q, Jing X, Liu J, Song D, Hu S, Liu L, Wang J. Removal of uranium(VI) ions from aqueous solution by magnetic cobalt ferrite/multiwalled carbon nanotubes composites. Chemical Engineering Journal, 2015, 273: 307–315
Wang Y, Wang Z, Gu Z, Yang J, Liao J, Yang Y, Liu N, Tang J. Uranium(VI) sorption on graphene oxide nanoribbons derived from unzipping of multiwalled carbon nanotubes. Journal of Radioanalytical and Nuclear Chemistry, 2015, 304(3): 1329–1337
Yuan D, Chen L, Xiong X, Yuan L, Liao S, Wang Y. Removal of uranium(VI) from aqueous solution by amidoxime functionalized superparamagnetic polymer microspheres prepared by a controlled radical polymerization in the presence of DPE. Chemical Engineering Journal, 2016, 285: 358–367
Titirici M, White R, Brun N, Budarin V, Su D, del Monte F, Clark J, MacLachlan M. Sustainable carbon materials. Chemical Society Reviews, 2015, 44(1): 250–290
Thakur K, Kandasubramanian B. Graphene and graphene oxidebased composites for removal of organic pollutants: A review. Journal of Chemical & Engineering Data, 2019, 64(3): 833–867
Terrones M, Botello-Méndez A, Campos-Delgado J, López-Uríasd F, Vega-Cantúd Y, Rodriguez-Macíasd F, Elias A, Cano-Márquez A, Charlier J, Terrones H. Graphene and graphite nanoribbons: Morphology, properties, synthesis, defects and applications. Nano Today, 2010, 5(4): 351–372
Sun Z, Chen D, Chen B, Kong L, Su M. Enhanced uranium(VI) adsorption by chitosan modified phosphate rock. Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 2018, 547: 141–147
Vakili M, Rafatullah M, Salamatinia B, Abdullah A, Ibrahim M, Tan K, Gholami Z, Amouzgar P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydrate Polymers, 2014, 113: 115–130
Aizat M, Aziz F. Chitosan Nanocomposite Application in Wastewater Treatments, Nanotechnology in Water and Wastewater Treatment. Amsterdam: Elsevier, 2019: 243–265
Zhang L, Zeng Y, Cheng Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. Journal of Molecular Liquids, 2016, 214: 175–191
Pan D, Fan Q, Fan F, Tang Y, Zhang Y, Wu W. Removal of uranium contaminant from aqueous solution by chitosan@attapulgite composite. Separation and Purification Technology, 2017, 177: 86–93
Xu C, Wang J, Yang T, Chen X, Liu X, Ding X. Adsorption of uranium by amidoximated chitosan-grafted polyacrylonitrile, using response surface methodology. Carbohydrate Polymers, 2015, 121: 79–85
Huang Z, Li Z, Zheng L, Zheng L, Zhou L, Chai Z,Wang X, Shi W. Interaction mechanism of uranium(VI) with three-dimensional graphene oxide-chitosan composite: Insights from batch experiments, IR, XPS and EXAFS spectroscopy. Chemical Engineering Journal, 2017, 328: 1066–1074
Samuel M, Shah S, Bhattacharya J, Subramaniam K, Singh N. Adsorption of Pb(II) from aqueous solution using a magnetic chitosan/graphene oxide composite and its toxicity studies. International Journal of Biological Macromolecules, 2018, 115: 1142–1150
Fan L, Luo C, Sun M, Li X, Qiu H. Highly selective adsorption of lead ions by water-dispersible magnetic chitosan/graphene oxide composites. Colloids and Surfaces. B, Biointerfaces, 2013, 103: 523–529
Elwakeel K, Atia A. Uptake of U (VI) from aqueous media by magnetic Schiff’s base chitosan composite. Journal of Cleaner Production, 2014, 70: 292–302
Wu P, Wang Y, Hu X W, Yuan D, Liu Y, Liu Z. Synthesis of magnetic graphene oxide nanoribbons composite for the removal of Th(IV) from aqueous solutions. Journal of Radioanalytical and Nuclear Chemistry, 2019, 319(3): 1111–1118
Samuel M, Bhattacharya J, Raj S, Santhanam N, Singh H, Singh N. Efficient removal of Chromium(VI) from aqueous solution using chitosan grafted graphene oxide (CS-GO) nanocomposite. International Journal of Biological Macromolecules, 2019, 121: 285–292
Hsan N, Dutta P, Kumar S, Bera R, Das N. Chitosan grafted graphene oxide aerogel: Synthesis, characterization and carbon dioxide capture study. International Journal of Biological Macromolecules, 2019, 125: 300–306
Anush S M, Chandan H, Vishalakshi B. Synthesis and metal ion adsorption characteristics of graphene oxide incorporated chitosan Schiff base. International Journal of Biological Macromolecules, 2019, 126: 908–916
Samuel M, Shah S, Subramaniyan V, Qureshi T, Bhattacharya J, Singh N. Preparation of graphene oxide/chitosan/ferrite nanocomposite for chromium(VI) removal from aqueous solution. International Journal of Biological Macromolecules, 2018, 119: 540–547
Mende M, Schwarz D, Steinbach C, Boldt R, Schwarz S. Simultaneous adsorption of heavy metal ions and anions from aqueous solutions on chitosan—investigated by spectrophotometry and SEM-EDX analysis. Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 2016, 510: 275–282
Yang A, Yang P, Huang C. Preparation of graphene oxide–chitosan composite and adsorption performance for uranium. Journal of Radioanalytical and Nuclear Chemistry, 2017, 313(2): 371–378
Hosseinzadeh H, Ramin S. Effective removal of copper from aqueous solutions by modified magnetic chitosan/graphene oxide nanocomposites. International Journal of Biological Macromolecules, 2018, 113: 859–868
El Rouby W, Farghali A, Sadek M, Khalil W. Fast removal of Sr(II) from water by graphene oxide and chitosan modified graphene oxide. Journal of Inorganic and Organometallic Polymers and Materials, 2018, 28(6): 2336–2349
Liao Y, Wang M, Chen D. Preparation of polydopamine-modified graphene oxide/chitosan aerogel for uranium(VI) adsorption. Industrial & Engineering Chemistry Research, 2018, 57(25): 8472–8483
Hyun S P, Davis J, Sun K, Hayes K. Uranium(VI) reduction by iron (II) monosulfide mackinawite. Environmental Science & Technology, 2012, 46(6): 3369–3376
Tripathi S, Roy A, Nair S, Durani S, Bose R. Removal of U (VI) from aqueous solution by adsorption onto synthesized silica and zinc silicate nanotubes: Equilibrium and kinetic aspects with application to real samples. Environmental Nanotechnology, Monitoring & Management, 2018, 10: 127–139
Tavengwa N, Cukrowska E, Chimuka L. Modeling of adsorption isotherms and kinetics of uranium sorption by magnetic ion imprinted polymers. Toxicological and Environmental Chemistry, 2016, 98(1): 1–12
Kapnisti M, Noli F, Misaelides P, Vourliasc G, Karfaridisc D, Hatzidimitrioua A. Enhanced sorption capacities for lead and uranium using titanium phosphates; sorption, kinetics, equilibrium studies and mechanism implication. Chemical Engineering Journal, 2018, 342: 184–195
Cai Y, Wu C, Liu Z, Zhang L, Chen L, Wang J, Wang X, Yang S, Wang S. Fabrication of a phosphorylated graphene oxide–chitosan composite for highly effective and selective capture of U(VI). Environmental Science. Nano, 2017, 4(9): 1876–1886
Liu W, Dai X, Bai Z, Wang Y, Yang Z, Zhang L, Xu L, Chen L, Li Y, Gui D, et al. Highly sensitive and selective uranium detection in natural water systems using a luminescent mesoporous metal–organic framework equipped with abundant Lewis basic sites: A combined batch, X-ray absorption spectroscopy, and first principles simulation investigation. Environmental Science & Technology, 2017, 51(7): 3911–3921
Li H, Zhai F, Gui D, Wang X, Wu C, Zhang D, Dai X, Deng H, Su X, Diwu J, et al. Powerful uranium extraction strategy with combined ligand complexation and photocatalytic reduction by postsynthetically modified photoactive metal-organic frameworks. Applied Catalysis B: Environmental, 2019, 254: 47–54
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 21601033, 21866006, 11875105, 21661003 and 11705027), Natural Science Foundation of Jiangxi Province (No. 20192BAB202007), Natural Science Funds for Distinguished Young Scholar of Jiangxi Province (No. 20171BCB23067), Open Project Foundation of Nuclear Technology Application Ministry of Education Engineering Research Center (East China University of Technology) (No. HJSJYB2016-6), Open Project Foundation of Stake key Laboratory of Nuclear Resources and Environment (East China University of Technology) (No. NRE1509), and Fundamental Science on Nuclear Wastes and Environmental Safety Laboratory (No. 16kfhk02)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, X., Wang, Y., Yang, J.O. et al. Synthesis of graphene oxide nanoribbons/chitosan composite membranes for the removal of uranium from aqueous solutions. Front. Chem. Sci. Eng. 14, 1029–1038 (2020). https://doi.org/10.1007/s11705-019-1898-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-019-1898-9