Abstract
Glomeruloid architecture is the least common Gleason 4 growth pattern in prostate adenocarcinoma. Its clinicopathological features and relation with cribriform architecture, which has been recognized as an adverse feature, remains to be established. Our objective was to investigate clinicopathological features of glomeruloid architecture in radical prostatectomies. We reviewed 1064 radical prostatectomy specimens and recorded Grade Group, pT-stage, margin status, Gleason pattern percentages, and growth patterns. Simple and complex glomerulations were distinguished by gland size and intraluminal cribriform protrusions. Clinical endpoint was biochemical recurrence-free survival. Glomerulations were identified in 365 (34%) specimens. In 472 Grade Group 2 patients, 210 (44%) had simple and 92 (19%) complex glomerulations. Complex glomerulations coincided with cribriform architecture more often than simple glomerulations (67% versus 52%; P = 0.01). Men with simple glomerulations had significantly lower prostate specific antigen (PSA) levels (9.7 versus 12.1 ng/ml; P = 0.03), percentage Gleason pattern 4 (19% versus 25%; P = 0.001), extra-prostatic extension (34% versus 50%; P = 0.01), and positive surgical margins (25% versus 39%; P = 0.04) than those with cribriform architecture. Extra-prostatic extension (37%) and positive surgical margins (30%) in men with complex glomerulations resembled those with simple glomeruloid rather than those with cribriform architecture. In multivariate Cox regression analysis adjusted for PSA, pT-stage, margin status, and lymph node metastases, cribriform architecture had independent predictive value for biochemical recurrence-free survival (hazard ratio (HR)) 1.9; 95% confidence interval (CI) 1.2–2.9; P = 0.004), while simple (HR 0.8; 95% CI 0.5–1.2; P = 0.26) and complex (HR 0.9; 95% CI 0.5–1.6; P = 0.67) glomerulations did not. Both simple and complex glomeruloid architecture are associated with better outcome than cribriform architecture in Grade Group 2 prostate cancer patients. Therefore, glomeruloid pattern and particularly complex glomerulations should not be classified as a cribriform growth pattern variant in radical prostatectomy specimens.
Similar content being viewed by others
Introduction
The Gleason score and Grade Group are the most important parameters for clinical outcome in prostate cancer patients [1, 2]. Gleason pattern 4 is a heterogeneous group of growth patterns including poorly formed, fused, glomeruloid and cribriform structures. The clinical importance of cribriform architecture in prostate cancer has been well established in recent years, as it is independently associated with disease progression and disease-specific death [3,4,5,6,7,8,9]. Glomeruloid growth pattern consists of dilated malignant glands with intraluminal cribriform protrusions, attached to one side of the gland wall, resembling a renal glomerulus [2]. Pacelli et al. first described this growth pattern in relation to tumor grade and stage [10]. Cribriform and glomeruloid growth patterns are often observed together and some have hypothesized that glomeruloid morphology might be a precursor of cribriform architecture [11, 12]. However, more recent studies indicate that glomeruloid pattern is associated with beneficial histopathological features and longer biochemical recurrence-free survival among Gleason score 7 prostate cancer patients [6, 13].
Interobserver studies have shown that glomeruloid architecture is one of the most reproducible growth patterns in prostate cancer grading [14, 15]. While interobserver agreement is excellent for small glomeruloid protrusions, no consensus exists on the classification of large glomeruloid structures as either glomeruloid or cribriform growth pattern [15]. Since some institutes use cribriform architecture as threshold for active surveillance in Grade Group 2 prostate cancer, distinction between glomeruloid and cribriform growth patterns might have major implications for patient management. Therefore, the aim of this study was to investigate the clinicopathological features and biochemical recurrence-free survival of prostate cancer patients with glomeruloid growth pattern who had undergone radical prostatectomy.
Methods
Patient selection
Patients who had undergone radical prostatectomy for prostate adenocarcinoma in three medical centers in The Netherlands between 2000 and 2017 were included in this study. In total, 854 patients were operated at Erasmus MC, University Medical Center, Rotterdam. In addition, patients from Leiden University Medical Center, Leiden (n = 96), and the Netherlands Cancer Institute, Amsterdam (n = 137) were selected for high-grade morphology (Grade Groups 3–5). We excluded men who had undergone hormonal, radiation, or viral therapy (n = 23) prior to operation [16]. Radical prostatectomy specimens were fixed in neutral-buffered formalin, after which they were sectioned transversely and completely embedded for diagnostic evaluation. All slides were available for pathology review. This study was approved by the institutional Medical Research Ethics Committee (MEC-2018–1614).
Pathologic evaluation
Radical prostatectomy specimens were reviewed by two investigators (EH, GJLHvL) in common sessions, blinded to clinical outcome. For each specimen the following features were recorded: Gleason score and Grade Group according to the World Health Organization and International Society of Urological Pathology (ISUP) 2014 guidelines, pT-stage according to the American Joint Committee on Cancer TNM 8th edition, surgical margin status, presence of individual growth patterns and intraductal carcinoma, and percentage of Gleason pattern 4 and 5 [2, 17]. The following Gleason 4 growth patterns were recognized: poorly formed, fused, glomeruloid, and cribriform glands [2, 18]. Furthermore, we distinguished two subgroups of glomeruloid growth pattern based on the architecture of intraluminal protrusions (Fig. 1). Simple glomeruloid architecture was defined as malignant glands with small to medium-sized solid intraluminal cell clusters with unilocular connection to the gland wall. Complex glomeruloid growth pattern had medium to large-sized intraluminal cribriform protrusions with unilocular connection to the gland wall; in some cases the gland wall connection was more extensive. The distinction between glomeruloid and cribriform architecture was arbitrarily made by the extent of gland wall connection, which occupied at least half of the inner glandular surface in cribriform growth and less than half in the glomeruloid pattern. In addition, we distinguished small and large cribriform growth patterns, the latter being defined as cribriform structures with a diameter more than twice the size of adjacent benign glands. Invasive cribriform Gleason pattern 4 was morphologically distinguished from intraductal carcinoma based on the following features: invasive cribriform prostate cancer had an irregular outline, interconnecting fields beyond preexistent gland architecture, or extension into extra-prostatic tissue. Intraductal carcinoma was morphologically identified if cribriform structures were continuous with preexistent glands or contained corpora amylacea. If invasive cribriform carcinoma and intraductal carcinoma could not be distinguished by morphological criteria alone, additional basal cell immunohistochemistry was performed. Cribriform glands completely lacking basal cell staining were categorized as invasive cribriform carcinoma. If basal cells were present sporadically, scattered, or continuously, the cribriform structures were classified as intraductal carcinoma. Gleason pattern 5 was considered as a tertiary pattern if it occupied less than 5% of the total tumor area [2, 18, 19]. Intraductal carcinoma and tertiary patterns were not incorporated in the Gleason score.
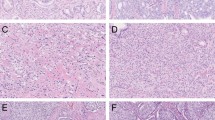
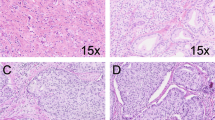
a Simple glomeruloid architecture with intraluminal cell clusters, 10×. b Complex glomeruloid architecture with large cribriform proliferations protruding into the lumen, 5×. c Simple glomeruloid architecture with vacuolated cytoplasm, therefore not classified as complex glomeruloid architecture, 20×. d Complex glomeruloid architecture (asterisk) based on a gland wall connection that occupies less than half of the inner gland surface, and simple glomeruloid architecture (arrows) with a small intraluminal protrusion, 15×. e Small invasive cribriform carcinoma, 10×. f Large invasive cribriform carcinoma showing expansive confluent fields, 5×.
Clinical follow-up
Postoperative clinical follow-up consisted of six-monthly, and later annual monitoring of serum prostate specific antigen (PSA) levels. Biochemical recurrence was defined as PSA levels ≥0.2 ng/ml measured at two consecutive points in time, at least 3 months apart with undetectable PSA levels after operation, or as PSA increase of >2.0 ng/ml when serum PSA had not declined to zero after the operation. Postoperative lymph node and distant metastases were confirmed by biopsy or multidisciplinary consensus.
Statistical analysis
Continuous variables with normal distribution were analyzed using the independent sample Student’s t test. Pearson’s chi squared (χ2) test was used for categorical parameters. Missing PSA values (n = 27) were imputed using the median PSA value. Biochemical recurrence-free survival and metastasis-free survival were analyzed using Cox proportional hazards regression and visualized by Kaplan–Meier curves. Statistics were performed using SPSS version 24 (IBM, Chicago, IL, USA). Results were considered significant when the two-sided P value was < 0.05.
Results
General patients characteristics
The cohort consisted of 1064 men with a median age of 65 years (interquartile range (IQR) 60–68) and median serum PSA level of 8.3 ng/ml (IQR 6.0–13.2). Median follow-up was 61 months (IQR 20–104). The cohort included 207 (20%) men with Grade Group 1, 472 (44%) with Grade Group 2, 126 (12%) with Grade Group 3, 140 (13%) with Grade Group 4, and 119 (11%) with Grade Group 5 prostate cancer. Pathological stage was distributed as follows: 582 (55%) pT2, 334 (31%) pT3a, 145 (14%) pT3b, and 3 (0.3%) pT4 tumors. Surgical margins were positive in 389 (37%) patients. Pelvic lymph node dissection was performed in 664 (62%) patients, of whom 64 (10%) had lymph node metastases.
Gleason 4 growth patterns
Poorly formed and fused glands were observed in 691 (65%) and 613 (58%) men, respectively. Invasive cribriform pattern was present in 519 (49%) men, 189 (18%) of whom had large expansive growth. Intraductal carcinoma was identified in 314 (30%) specimens. In total, 569 (54%) men had invasive cribriform and/or intraductal carcinoma, 190 (33%) of whom had large invasive cribriform growth. Glomeruloid growth was the least frequent Gleason 4 pattern, being present in 365 (34%) men. It was the single Gleason 4 pattern in only 10 (1%) men. Simple glomeruloid glands were present in 352 (33%) men and complex glomeruloid glands in 154 (15%) men. Among patients with glomeruloid architecture, 211 (58%) had simple glomerulations only, 13 (4%) had complex glomerulations only, and 141 (38%) had both simple and complex glomeruloid glands. Simple and complex glomeruloid patterns had concomitant invasive and/or intraductal cribriform carcinoma in 128/211 (61%) and 121/154 (79%) cases (P < 0.001), respectively. Complex glomeruloid growth did not coincide more often with large compared with small cribriform growth (P = 0.26).
Simple glomerulations were present in 212 (45%) Grade Group 2, 56 (44%) Grade Group 3, 47 (34%) Grade Group 4, and 28 (24%) Grade Group 5 tumors. In 9 (4%) men with Grade Group 1 tumors, simple glomerulations were present as tertiary Gleason pattern 4. Complex glomerulations were observed in 92 (19%) men with Grade Group 2, 34 (27%) with Grade Group 3, 15 (11%) with Grade Group 4, and 13 (11%) with Grade Group 5 tumors. In none of the cases with Grade Group 1, complex glomerulations were present as tertiary pattern. Simple and complex glomerulations were observed significantly more often in Grade Groups 2 and 3 patients compared with Grade Groups 4 and 5 patients (P = 0.05).
Glomeruloid architecture in Grade Group 2 prostate cancer
Since clinical impact of glomeruloid growth pattern classification is most relevant for Grade Group 2 prostate cancer patients, we performed further analyses in this subpopulation of 472 men. Of these, 216 (46%) had glomerulations: 212 (45%) had simple and 92 (20%) complex glomeruloid structures. Simple glomerulations only were present in 124 (57%) men, complex glomerulations only in 4 (2%) men, and both patterns occurred concurrently in 88 (41%) cases. Invasive and/or intraductal cribriform carcinoma was present in 252 (53%) men with Grade Group 2 prostate cancer, 34 (13%) of whom had large invasive cribriform carcinoma. Glomeruloid architecture was associated with presence of invasive and/or intraductal cribriform carcinoma, as 126/216 (58%) men with glomerulations had coexistent cribriform architecture compared with 126/256 (49%) men without glomerulations (P = 0.05). Complex glomeruloid structures (62/92, 67%) were more frequently concomitant with invasive and/or intraductal cribriform carcinoma than simple glomerulations (64/124, 52%, P = 0.01).
Further analyses on glomeruloid and cribriform growth patterns were performed in four subgroups (Table 1), to investigate the relation between glomeruloid and cribriform architecture. These subgroups consisted of men with neither glomeruloid nor cribriform architecture, thus with poorly formed and fused glands only (n = 130, group A), men with simple glomeruloid pattern without complex and/or cribriform architecture (n = 60, group B), men with complex glomeruloid pattern without invasive and/or intraductal cribriform carcinoma (n = 30, group C), and men with invasive and/or intraductal cribriform carcinoma regardless of presence of glomerulations (n = 252, group D). Patients with invasive cribriform and/or intraductal carcinoma (group D) had significantly higher percentage Gleason pattern 4 (25% versus 18%; P < 0.001), pT-stage (50% versus 36% pT3; P = 0.003), and positive surgical margin rates (39% versus 27%; P = 0.02) than those with poorly formed and fused glands only (group A). Men with simple glomerulations (group B) had similar PSA levels (9.7 versus 9.1 ng/ml; P = 0.6), percentage Gleason pattern 4 (19% versus 18%; P = 0.6), pT-stage (34% versus 36% pT3; P = 0.8), and positive surgical margins (25% versus 27%; P = 0.7) to men with poorly formed and fused glands only (group A). Compared with men with invasive cribriform and/or intraductal carcinoma (group D), men with simple glomerulations (group B) had significantly lower PSA levels (9.7 versus 12.1 ng/ml; P = 0.03), percentage Gleason pattern 4 (19% versus 25%; P = 0.001), pT-stage (34% versus 50% pT3; P = 0.01), and positive surgical margins (25% versus 39%; P = 0.04). Although men with complex glomeruloid glands only (group C) had higher PSA levels (13.1 versus 9.7 ng/ml; P = 0.05) than men with simple glomeruloid growth pattern (group B), percentage Gleason pattern 4 (20% versus 19%; P = 0.6), presence of tertiary Gleason pattern 5 (both 10%; P = 1.0), pT-stage (37% versus 34% pT3; P = 0.8), and surgical margin status (30% versus 25%; P = 0.6) were similar. Patients with complex glomeruloid glands (group C) had similar PSA levels (13.1 versus 12.1 ng/ml; P = 0.7) to those with invasive cribriform and/or intraductal carcinoma (group D), but its percentage Gleason pattern 4 was significantly lower (20% versus 25%; P = 0.04). Although the complex glomeruloid sample size (n = 30) was too low for reliable statistical analysis, percentage of Gleason pattern 4, presence of tertiary pattern 5, pT-stage, and positive surgical margin status resembled simple glomerulations (group B) rather than cribriform architecture (group D). Among patients with invasive and/or intraductal cribriform carcinoma (group D), no significant differences were found in PSA, pT-stage, percentage Gleason pattern 4, presence of tertiary Gleason pattern 5, and surgical margin status between those with and without glomeruloid architecture (data not shown).
Clinical outcome of Grade Group 2 patients
Median follow-up of Grade Group 2 patients was 57 months (IQR 14–99). Biochemical recurrence occurred in 22/130 (17%) men with poorly formed and fused glands only (group A), in 8/60 (13%) men with simple glomerulations (group B), in 4/30 (13%) men with complex glomerulations (group C), and in 73/252 (29%) men with cribriform architecture (group D). Survival curves are shown in Fig. 2. No statistically significant difference in biochemical recurrence-free survival was found between men with poorly formed and fused glands only (group A), simple glomeruloid glands (group B), or complex glomeruloid glands (group C, log rank P = 0.38). Patients with invasive and/or intraductal cribriform architecture (group D) had significantly shorter biochemical recurrence-free survival (log rank P < 0.001) than patients with simple (group B, P = 0.006) and complex glomeruloid glands (group C, P = 0.05).
Kaplan–Meier curves show biochemical recurrence-free survival in Grade Group 2 patients with cribriform and/or glomeruloid architecture, stratified for subgroups: men with neither glomeruloid nor cribriform architecture (group A), men with simple glomeruloid pattern without complex and/or cribriform architecture (group B), men with complex glomeruloid pattern without invasive and/or intraductal cribriform carcinoma (group C), and men with invasive and/or intraductal cribriform carcinoma regardless of presence of glomerulations (group D).
Cox regression analysis showed that intraductal carcinoma (hazard ratio (HR) 2.7, 95% confidence interval (CI) 1.8–4.0, P < 0.001), small invasive cribriform carcinoma (HR 1.9, 95% CI 1.3–3.0, P = 0.002), and large invasive cribriform carcinoma (HR 6.3, 95% CI 3.6–11.1, P < 0.001) were associated with shorter biochemical recurrence-free survival in univariate analysis (Table 2). Adjusted for PSA level, pT-stage, surgical margin, and pelvic lymph node metastases, large invasive cribriform carcinoma remained an independent predictor for biochemical recurrence (HR 3.8, 95% CI 2.1–6.8, P < 0.001) in multivariable analysis. Simple (HR 0.8; 95% CI 0.6–1.4; P = 0.64) and complex (HR 0.6; 95% CI 0.4–1.2; P = 0.19) glomeruloid growth patterns were not associated with biochemical recurrence-free survival in univariate or multivariable analysis. Metastases (n = 17) and disease-specific death (n = 3) only occurred in patients with presence of invasive cribriform and/or intraductal carcinoma, and not in other subgroups.
Discussion
In this study we investigated the clinicopathological features of glomeruloid Gleason pattern 4 architecture. Overall glomeruloid architecture was present in 34% of radical prostatectomy specimens. In Grade Group 2 patients, simple glomeruloid was seen in 45% and complex glomeruloid growth in 20% of men. Men with simple glomeruloid glands only had similar clinicopathological characteristics to those with poorly formed and fused glands. Although patients with complex glomeruloid glands had higher PSA levels than men with poorly formed, fused or simple glomeruloid glands, no significant difference was observed for pT-stage, percentage Gleason pattern 4, or biochemical recurrence-free survival. Biochemical recurrence and metastasis occurred significantly more often in patients with invasive cribriform and/or intraductal carcinoma. Despite their morphological resemblance, men with complex glomerulations had better outcome than those with cribriform architecture. Therefore, complex glomeruloid pattern should not be classified as a variant of the more aggressive cribriform growth pattern in radical prostatectomy specimens.
Pathological grading of the glomeruloid growth pattern has been uncertain for a long time. At the 2005 ISUP conference, no consensus was reached on grading glomeruloid pattern due to lack of scientific evidence for its prognostic value [20]. Lotan and Epstein studied 45 prostate cancer biopsies with glomeruloid features and found they were surrounded by Gleason pattern 4 structures in 80% and Gleason pattern 5 in 4% of cases [11]. The authors noted that half of the glomeruloid structures were accompanied by cribriform pattern, which is in concordance with our findings. For this reason it was unanimously consented at the 2014 ISUP conference that glomeruloid glands should be assigned Gleason pattern 4, regardless of morphology [2].
Since the increased awareness of the dismal outcome of cribriform Gleason pattern 4, glomeruloid growth pattern has also been included in clinicopathological studies. Among 350 radical prostatectomies with Gleason score 7 prostate cancer, Choy et al. found that patients with glomeruloid pattern had improved 5-year biochemical recurrence-free survival in multivariable analysis, but had shorter survival than those with Gleason score 6 [13]. Glomeruloid growth pattern was associated with improved, although not statistically significant, metastasis-free survival of Gleason score 7 men in our previous radical prostatectomy study [6]. However, both studies did not focus on glomeruloid architecture specifically and were performed on a Gleason score 7 cohort. The study of Kweldam et al. had a case-control study design and used metastasis-free survival as endpoint. We investigated glomeruloid architecture in a larger cohort of Grade Groups 1–5 prostate cancer and distinguished simple and complex glomerulations, which was not done in previous manuscripts. In the current study, clinical outcome of patients with glomeruloid architecture was better than of those with cribriform architecture, and did not differ from those with poorly formed and fused glands only. Statistical analyses were hampered by the fact that glomeruloid architecture is rarely observed as single Gleason pattern 4, mostly coexisting with other growth patterns.
Glomeruloid architecture is not a homogeneous Gleason pattern 4 subgroup. As previously reported by Lotan and Epstein, most glomerulations consisted of relatively small dilated glands, but some contain larger glomeruloid protrusions [11]. Furthermore, a subset of glomerulations has fibrovascular cores. This is in line with three-dimensional renderings of glomeruloid structures, which revealed an interconnecting network of tubules resembling Gleason pattern 3 glands with nodular epithelial proliferations near tubular branching points, or markedly curved tubules with small fibrovascular cores [21]. While glomeruloid architecture is the most reproducible Gleason 4 growth pattern, interobserver variability exists for classifying glomeruloid structures with larger intraluminal cribriform protrusions [15]. In this case, classification as either glomeruloid or cribriform architecture is uncertain, because the extent of cribriform intraluminal gland wall attachment has not been defined. Among Grade Group 2 prostate cancer patients, two-thirds of specimens with complex glomeruloid pattern had concomitant cribriform pattern, which might suggest that both patterns are related. Complex cribriform pattern for instance could be a tangential sectioning of cribriform architecture expanding into malignant tubules or might represent a precursor lesion. While designation of these structures as either cribriform or glomeruloid does not have clinical relevance if they coexist with cribriform architecture, their distinction might be important in diagnostic biopsies without cribriform structures. Active surveillance is mostly offered to men with biopsy Grade Group 1 disease, but some surveillance protocols are also including men with Grade Group 2 prostate cancer without cribriform architecture and/or low Gleason pattern 4 percentages [22,23,24,25,26,27]. Therefore, classifying complex glomeruloid structures might directly affect clinical decision-making. Our current findings in radical prostatectomy specimens indicate that men with complex glomeruloid structures without concomitant cribriform growth have better outcome than those with cribriform architecture. Therefore, at this moment insufficient evidence exists for classifying complex glomeruloid pattern as cribriform Gleason pattern 4.
The strong point of this study was the detailed histological review of a large cohort of radical prostatectomy specimens. In this study the distinction between glomeruloid and cribriform architecture was arbitrarily made by the extent of gland wall connection, which occupied at least half of the inner glandular surface in cribriform growth and less in the glomeruloid pattern. No standard definition of complex glomeruloid pattern has been formulated yet. Therefore, it is important that future studies define the morphological criteria for distinguishing complex glomeruloid and cribriform growth patterns. Some pathologists might have interpreted the complex glomeruloid cases as cribriform, however our current results do not provide evidence for this classification. Finally, this study was limited by its retrospective design and relatively short follow-up of 57 months.
In conclusion, glomeruloid architecture is observed in 34% of radical prostatectomy specimens. Patients with simple glomeruloid glands have similar clinicopathological characteristics and biochemical recurrence-free survival as poorly formed and fused glands in Grade Group 2 prostate cancer. Although complex glomeruloid glands are more often coexistent with cribriform growth pattern, biochemical recurrence and metastasis occurred significantly less frequently in this subgroup. Therefore, complex glomeruloid architecture does not classify as a cribriform Gleason 4 growth pattern in radical prostatectomy specimens. Further studies are needed to address the significance of glomeruloid architecture in prostate biopsies.
References
Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50:125–8.
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–52.
Iczkowski KA, Torkko KC, Kotnis GR, Wilson RS, Huang W, Wheeler TM, et al. Digital quantification of five high-grade prostate cancer patterns, including the cribriform pattern, and their association with adverse outcome. Am J Clin Pathol. 2011;136:98–107.
Kimura K, Tsuzuki T, Kato M, Saito AM, Sassa N, Ishida R, et al. Prognostic value of intraductal carcinoma of the prostate in radical prostatectomy specimens. Prostate. 2014;74:680–7.
Kir G, Sarbay BC, Gumus E, Topal CS. The association of the cribriform pattern with outcome for prostatic adenocarcinomas. Pathol Res Pr. 2014;210:640–4.
Kweldam CF, Wildhagen MF, Steyerberg EW, Bangma CH, van der Kwast TH, van Leenders GJ. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod Pathol. 2015;28:457–64.
Trudel D, Downes MR, Sykes J, Kron KJ, Trachtenberg J, van der Kwast TH. Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur J Cancer. 2014;50:1610–6.
Hollemans E, Verhoef EI, Bangma CH, Rietbergen J, Helleman J, Roobol MJ, et al. Large cribriform growth pattern identifies ISUP grade 2 prostate cancer at high risk for recurrence and metastasis. Mod Pathol. 2019;32:139–46.
Zhang XRLL, Cheville J. Gleason Grade 4 expansile cribriform pattern is associated with poor prognosis in prostate cancer. In: The 107th Annual Meeting of the United States and Canadian Academy of Pathology. Vancouver, BC, Canada: USCAP; 2018. Abstract number 114.
Pacelli A, Lopez-Beltran A, Egan AJ, Bostwick DG. Prostatic adenocarcinoma with glomeruloid features. Hum Pathol. 1998;29:543–6.
Lotan TL, Epstein JI. Gleason grading of prostatic adenocarcinoma with glomeruloid features on needle biopsy. Hum Pathol. 2009;40:471–7.
Tolkach Y, Kristiansen G. Cribriform and glomeruloid acinar adenocarcinoma of the prostate-an intratumoural intraductal carcinoma? Histopathology. 2019;74:804–8.
Choy B, Pearce SM, Anderson BB, Shalhav AL, Zagaja G, Eggener SE, et al. Prognostic significance of percentage and architectural types of contemporary Gleason pattern 4 prostate cancer in radical prostatectomy. Am J Surg Pathol. 2016;40:1400–6.
Egevad L, Ahmad AS, Algaba F, Berney DM, Boccon-Gibod L, Comperat E, et al. Standardization of Gleason grading among 337 European pathologists. Histopathology. 2013;62:247–56.
Kweldam CF, Nieboer D, Algaba F, Amin MB, Berney DM, Billis A, et al. Gleason grade 4 prostate adenocarcinoma patterns: an interobserver agreement study among genitourinary pathologists. Histopathology. 2016;69:441–9.
van der Linden RR, Haagmans BL, Mongiat-Artus P, van Doornum GJ, Kraaij R, Kadmon D, et al. Virus specific immune responses after human neoadjuvant adenovirus-mediated suicide gene therapy for prostate cancer. Eur Urol. 2005;48:153–61.
Buyyounouski MK, Choyke PL, McKenney JK, Sartor O, Sandler HM, Amin MB, et al. Prostate cancer—major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:245–53.
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol. 2016;70:106–19.
Epstein JI, Amin MB, Reuter VE, Humphrey PA. Contemporary Gleason grading of prostatic carcinoma: an update with discussion on practical issues to implement the 2014 International Society Of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol. 2017;41:1–7.
Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL, Committee IG. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–42.
Verhoef EI, van Cappellen WA, Slotman JA, Kremers GJ, Ewing-Graham PC, Houtsmuller AB, et al. Three-dimensional analysis reveals two major architectural subgroups of prostate cancer growth patterns. Mod Pathol. 2019;32:1032–41.
Cooperberg MR, Cowan JE, Hilton JF, Reese AC, Zaid HB, Porten SP, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol. 2011;29:228–34.
Gearman DJ, Morlacco A, Cheville JC, Rangel LJ, Karnes RJ. Comparison of pathological and oncologic outcomes of favorable risk gleason score 3 + 4 and low risk Gleason score 6 prostate cancer: considerations for active surveillance. J Urol. 2018;199:1188–95.
Klotz L. Active surveillance and focal therapy for low-intermediate risk prostate cancer. Transl Androl Urol. 2015;4:342–54.
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7.
Lee H, Lee IJ, Byun SS, Lee SE, Hong SK. Favorable Gleason 3 + 4 prostate cancer shows comparable outcomes with Gleason 3 + 3 prostate cancer: implications for the expansion of selection criteria for active surveillance. Clin Genitourin Cancer. 2017;15:1117–22.
Morlacco A, Cheville JC, Rangel LJ, Gearman DJ, Karnes RJ. Adverse disease features in Gleason score 3 + 4 “favorable intermediate-risk” prostate cancer: implications for active surveillance. Eur Urol. 2017;72:442–7.
Acknowledgements
This study was generously supported by a grant from the Jaap Schouten Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hollemans, E., Verhoef, E.I., Bangma, C.H. et al. Clinicopathological characteristics of glomeruloid architecture in prostate cancer. Mod Pathol 33, 1618–1625 (2020). https://doi.org/10.1038/s41379-020-0507-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0507-2