Abstract
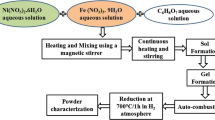
A pure crystalline γ-FeNi alloy powder was fabricated through the one-step process of sol–gel combustion. An experiment was conducted in inert atmosphere with the hexamethylenetetramine (HMTA) used as an oxygen-free fuel and metal nitrites as oxidizers. It was demonstrated that fuel-to-oxidizer ratio is a critical parameter to produce pure γ-FeNi alloy. A concentration of the fuel necessary for the alloy synthesis is three times higher (φ = 3) than at the stochiometric molar ratio. The ~1100 K maximum temperature, 830 K/s heating and 170 K/s cooling rates and the total reaction time of ~3 s are characteristics of the optimized sol–gel combustion synthesis of the γ-FeNi alloy powder. A mechanism of the alloy formation through the coordination metals—HMTA complex was proposed and experimentally verified. Microstructural and magnetic properties of the synthesized material are also investigated and discussed.

Highlights
-
Seconds long synthesis of pure alloy in a combustion wave.
-
Controllability issues and mechanism of combustion processes.
-
Fundamental correlations between fuel and oxidizer ratio in alloy synthesis.
-
Chemical approach to the experiment modification.
-
Verification of the theoretical prediction for the fast, high-temperature combustion synthesis.










Similar content being viewed by others
References
Wu H, Zeng M, Li Z, Zhu X, Tian C, Xia C, He L, Dai S (2019) Coupling FeNi alloys and hollow nitrogen-enriched carbon frameworks leads to high-performance oxygen electrocatalysts for rechargeable zinc-air batteries. Sustain Energy Fuels. https://doi.org/10.1039/c8se00362a
Liu Z, Yu H, Dong B, Yu X, Feng L (2018) Electrochemical oxygen evolution reaction efficiently boosted by thermal-driving core-shell structure formation in nanostructured FeNi/S, N-doped carbon hybrid catalyst. Nanoscale. https://doi.org/10.1039/c8nr05587d
Liu L, Yan F, Li K, Zhu C, Xie Y, Zhang X, Chen Y (2019) Ultrasmall FeNi 3 N particles with an exposed active (110) surface anchored on nitrogen-doped graphene for multifunctional electrocatalysts. J Mater Chem A. https://doi.org/10.1039/c8ta10083g
Tang X, Li L, Shen B, Wang C (2013) Halloysite-nanotubes supported FeNi alloy nanoparticles for catalytic decomposition of toxic phosphine gas into yellow phosphorus and hydrogen. Chemosphere. https://doi.org/10.1016/j.chemosphere.2013.02.010
Salati A, Ramazani A, Almasi Kashi M (2019) Deciphering magnetic hyperthermia properties of compositionally and morphologically modulated FeNi nanoparticles using first-order reversal curve analysis. Nanotechnology. https://doi.org/10.1088/1361-6528/aae7f3
Dong A, Zhiyi Z, Yanhui W, Shuaishuai C, Yaqing L (2019) The distinctly enhanced electromagnetic wave absorption properties of FeNi/rGO nanocomposites compared with pure FeNi alloys. J Supercond Nov Magn 32:385–392. https://doi.org/10.1007/s1094
Golchinvafa S, Masoudpanah SM (2019) Magnetic and microwave absorption properties of FeNi3/NiFe2O4 composites synthesized by solution combustion method. J Alloy Compd 787:390–396. https://doi.org/10.1016/j.jallcom.2019.02.039
Ma Y, Dai X, Liu M, Yong J, Qiao H, Jin A, Li Z, Huang X, Wang H, Zhang X (2016) Strongly coupled FeNi alloys/NiFe2O4@carbonitride layers-assembled microboxes for enhanced oxygen evolution reaction. ACS Appl Mater Interfaces. 8:34396–34404
Cao D, Song Y, Pan L, Du H, Feng H, Zhao C, Li Q, Xu J, Li S, Liu Q, Wang J (2019) Influence of the phases structure on the acoustic and optical modes ferromagnetic resonance of FeNi stripe domain films. J Magn Magn Mater. https://doi.org/10.1016/j.jmmm.2018.11.094
Yan F, Wang Y, Li K, Zhu C, Gao P, Li C, Zhang X, Chen Y (2017) Highly stable three-dimensional porous nickel-iron nitride nanosheets for full water splitting at high current densities. Chem. A Eur J. https://doi.org/10.1002/chem.201701662
Chandrasekhar PS, Seo YH, Noh YJ, Na SI (2019) Room temperature solution-processed Fe doped NiOx as a novel hole transport layer for high efficient perovskite solar cells. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2019.03.164
Ghiami S, Nasseri MA, Allahresani A, Kazemnejadi M (2019) FeNi3@SiO2 nanoparticles: an efficient and selective heterogeneous catalyst for the epoxidation of olefins and the oxidation of sulfides in the presence of meta-chloroperoxybenzoic acid at room temperature. React Kinet Mech Catal. https://doi.org/10.1007/s11144-018-1479-9
Srakaew N, Jantaratana P, Nipakul P, Sirisathitkul C (2017) Structural and magnetic properties of FexNi100−x alloys synthesized using Al as a reducing metal. J Magn Magn Mater. https://doi.org/10.1016/j.jmmm.2017.04.018
Lu X, Liang G, Zhang Y (2007) Synthesis and characterization of magnetic FeNi3 particles obtained by hydrazine reduction in aqueous solution. Mater Sci Eng B Solid-State Mater Adv Technol. https://doi.org/10.1016/j.mseb.2007.01.055
Jia J, Yu JC, Wang YXJ, Chan KM (2010) Magnetic nanochains of FeNi3 prepared by a template-free microwave-hydrothermal method. ACS Appl Mater Interfaces. https://doi.org/10.1021/am100410r
Han T, Xu C, Chen H (2019) Simple synthesis of novel mushroom-like FeNi3 microstructures by a hydrothermal reduction, Mater Res Innov. https://doi.org/10.1080/14328917.2017.1362509
Ma T, Yuan M, Islam SM, Li H, Ma S, Sun G, Yang X (2016) FeNi3 alloy nanocrystals grown on graphene: controllable synthesis, in-depth characterization and enhanced electromagnetic performance. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2016.03.243
Liu J, Long Y, Bai D, Sun H, Zhang H, Long K, Yan T (2019) Magnetic properties of FeNi alloys for high-temperature thermomagnetic power generation. AIP Adv 9:045227. https://doi.org/10.1063/1.5086411
Dios M, Gonzalez Z, Alvaredo P, Bermejo R, Gordo E, Ferrari B (2017) Novel colloidal approach for the microstructural improvement in Ti(C,N)/FeNi cermets. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2017.07.034
Jartych E, Zurawicz JK, Oleszak D, Pȩkala M (2000) X-ray diffraction, magnetization and Mössbauer studies of nanocrystalline Fe-Ni alloys prepared by low- And high-energy ball milling. J Magn Magn Mater. https://doi.org/10.1016/S0304-8853(99)00543-0
Zhou R, Zhang Y, Zhu H, Mao M, Shen J, Dai B, Ren Y (2019) Tunable resonance frequency of wavelike FeNi nanofilm using a double-layer structure. J Magn Magn Mater. https://doi.org/10.1016/j.jmmm.2019.04.014
Varma A, Mukasyan AS, Rogachev AS, Manukyan KV (2016) Solution combustion synthesis of nanoscale materials. Chem Rev. https://doi.org/10.1021/acs.chemrev.6b00279
Khaliullin SM, Zhuravlev VD, Bamburov VG, Khort AA, Roslyakov SI, Trusov GV, Moskovskikh DO (2019) Effect of the residual water content in gels on solution combustion synthesis temperature. J Sol-Gel Sci Technol. https://doi.org/10.1007/s10971-019-05189-8
Kumar A, Wolf EE, Mukasyan AS (2011) Solution combustion synthesis of metal nanopowders: copper and copper/nickel alloys. AIChE J. https://doi.org/10.1002/aic.12537
Manukyan KV, Cross A, Roslyakov S, Rouvimov S, Rogachev AS, Wolf EE, Mukasyan AS (2013) Solution combustion synthesis of nano-crystalline metallic materials: mechanistic studies. J Phys Chem C 117. https://doi.org/10.1021/jp408260m
Khomenko IO, Mukasyan AS, Ponomaryev VI, Borovinskaya IP, Merzhanov AG (1993) Dynamics of phase forming processes in the combustion of metalgas systems. Combust Flame 92:201–208. https://doi.org/10.1016/0010-2180(93)90032-X
Shiryaev AA (1995) Thermodynamics of SHS Processes: Advanced Approach, Int. J. Self-Propagating High Temp Synth. https://doi.org/10.1017/CBO9781107415324.004
Cullity BD, Stock SR (2001) Elements of X-ray diffraction, 3rd edition. New York: Prentice-Hall
Leslie-Pelecky DL, Rieke RD (1996) Magnetic properties of nanostructured materials, Chem. Mater. https://doi.org/10.1021/cm960077f
Wack M, Volk M, Wei Q (2018) Magnetic properties of the iron–nickel system: pressure, composition, and grain size. https://doi.org/10.1007/978-3-319-64292-5_14
Liu Y, Qin M, Zhang L, Huang M, Li S, Jia B, Zhang D, Qu X (2014) Solution combustion synthesis of nanocrystalline Fe-50%Ni alloy powder. Powder Technol. https://doi.org/10.1016/j.powtec.2014.07.003
Kumar A, Wolf EE, Mukasyan AS (2011) Solution combustion synthesis of metal nanopowders: nickel-reaction pathways. AIChE J. https://doi.org/10.1002/aic.12416
Jadhav LD, Patil SP, Chavan AU, Jamale AP, Puri VR (2011) Solution combustion synthesis of Cu nanoparticles: a role of oxidant-to-fuel ratio. Micro Nano Lett. https://doi.org/10.1049/mnl.2011.0372
Deshpande K, Mukasyan A, Varma A (2004) Direct synthesis of iron oxide nanopowders by the combustion approach: Reaction mechanism and properties. Chem Mater. https://doi.org/10.1021/cm040061m
Kumar A, Miller JT, Mukasyan AS, Wolf EE (2013) In situ XAS and FTIR studies of a multi-component Ni/Fe/Cu catalyst for hydrogen production from ethanol. Appl Catal A Gen. https://doi.org/10.1016/j.apcata.2013.07.032
Liu Y, Li Qin M, Zhang L, Rui Jia B, Qi Chen P, Zhi Zhang D, Hui Qu X (2015) Solution combustion synthesis of Fe-Ni-Y2O3 nanocomposites for magnetic application. J Cent South Univ. https://doi.org/10.1007/s11771-015-2490-1
Mukasyan AS, Roslyakov S, Pauls JM, Gallington LC, Orlova T, Liu X, Dobrowolska M, Furdyna JK, Manukyan KV (2019) Nanoscale metastable σ-Fe3N ferromagnetic materials by self-sustained reactions. Inorg Chem. https://doi.org/10.1021/acs.inorgchem.8b03553
Afanasiev P, Chouzier S, Czeri T, Pilet G, Pichon C, Roy M, Vrinat M (2008) Nickel and cobalt hexamethylentetramine complexes (NO3)2Me(H2O)6(HMTA)2·4H2O (Me = Co2+, Ni2+): New molecular precursors for the preparation of metal dispersions. Inorg Chem. https://doi.org/10.1021/ic7013013
Il’chenko NI (1972) Influence of Metallic Additives on the Reduction of Solid Oxides. Russ Chem Rev. https://doi.org/10.1070/rc1972v041n01abeh002029
Kaihua J, Shuhong B (2018) Coordination compounds of hexamethylenetetramine with metal salts: a review, Johnson Matthey Technol Rev. https://doi.org/10.1595/205651317X696621
Ndifon PT, Agwara MO, Paboudam AG, Yufanyi DM, Ngoune J, Galindo A, Álvarez E, Mohamadou A (2009) Synthesis, characterisation and crystal structure of a cobalt(II)- hexamethylenetetramine coordination polymer. Transit Met Chem. https://doi.org/10.1007/s11243-009-9257-1
Pirali O, Boudon V, Carrasco N, Dartois E (2014) Rotationally resolved IR spectroscopy of hexamethylenetetramine (HMT) C6N4H12. Astron Astrophys. https://doi.org/10.1051/0004-6361/201322660
Tong HJ, Reid JP, Dong JL, Zhang YH (2010) Observation of the crystallization and supersaturation of mixed component NaNO3-Na2SO4 droplets by FTIR-ATR and Raman spectroscopy. J Phys Chem A. https://doi.org/10.1021/jp1080548
Goebbert DJ, Garand E, Wende T, Bergmann R, Meijer G, Asmis KR, Neumark DM (2009) Infrared spectroscopy of the microhydrated nitrate ions NO3(H2O). J Phys Chem A. https://doi.org/10.1021/jp9017103
Melnikov P, Nascimento VA, Arkhangelsky IV, Zanoni Consolo LZ, Oliveira LCS (2014) Thermal decomposition mechanism of iron(III) nitrate and characterization of intermediate products by the technique of computerized modeling. J Therm Anal Calorim. https://doi.org/10.1007/s10973-013-3339-1
Max JJ, Chapados C (2009) Isotope effects in liquid water by infrared spectroscopy. III. H2O and D2O spectra from 6000 to 0 cm-1. J Chem Phys. https://doi.org/10.1063/1.3258646
Eisenberg D, Kauzmann W (2007) The structure and properties of water. https://doi.org/10.1093/acprof:oso/9780198570264.001.0001
Nakagawa I, Shimanouchi T (1964) Infrared absorption spectra of aquo complexes and the nature of co-ordination bonds. Spectrochim Acta. https://doi.org/10.1016/0371-1951(64)80040-0
Vdovenko VM, (ed) (1964) Spectroscopic methods in the chemistry of complex compounds, Chemistry, Moskow
JL Mi, Nørby P, Bremholm M, Becker J, Iversen BB (2015) The formation mechanism of bimetallic PtRu alloy nanoparticles in solvothermal synthesis. Nanoscale. https://doi.org/10.1039/c5nr04459f
Afanasiev P (2002) New single source route to the molybdenum nitride Mo2N. Inorg Chem. https://doi.org/10.1021/ic025564d
Dhiman P, Batoo KM, Kotnala RK, Chand J, Singh M (2013) Room temperature ferromagnetism and structural characterization of Fe, Ni co-doped ZnO nanocrystals. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2013.09.144
Cao J, Qin Y, Li M, Zhao S, Li J (2014) Sol–gel combustion synthesis of magnetic MnFe2O4 oxide and FeNi alloy: product dependence on the reduction ability. Appl Phys A Mater Sci Process. https://doi.org/10.1007/s00339-014-8611-0
Acknowledgements
The work was carried out with financial support from the Ministry of Education and Science of the Russian Federation in the framework of Increase Competitiveness Program of NUST «MISiS» (№ К4-2018-016), implemented by a governmental decree dated 16th of March 2013, N211.
Funding
This study was funded by Ministry of Education and Science of the Russian Federation (grant number (№ К4-2018-016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yermekova, Z., Roslyakov, S.I., Kovalev, D.Y. et al. One-step synthesis of pure γ-FeNi alloy by reaсtive sol–gel combustion route: mechanism and properties. J Sol-Gel Sci Technol 94, 310–321 (2020). https://doi.org/10.1007/s10971-020-05252-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05252-9