Abstract
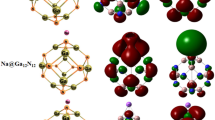
Performances of \(\hbox {C}_{30}\) and \(\hbox {Ge}_{30}\) nanocages for anode electrodes in metal-ion battery (MI-B) are studied. Abilities of halogens (F, Br and Cl) adsorption on \(\hbox {C}_{30}\) and \(\hbox {Ge}_{30}\) potential for anode electrodes of MI-Bs were investigated. Gibbs free energy, voltage of cell, adsorption energy and orbital energy values of studied complexes were calculated and were compared. Results displayed the \(V_{\mathrm{cell}}\) of \(\hbox {K-Ge}_{30}\) was higher than Na- and \(\hbox {Li-Ge}_{30}\) 0.15 and 0.29 V. \(V_{\mathrm{cell}}\) of K, Na and Li on \(\hbox {Ge}_{30}\) were higher than \(\hbox {C}_{30}\) 0.18, 0.17 and 0.15 V. The \(\hbox {G}_{\mathrm{ad}}\) of halogens (F, Br and Cl) on \(\hbox {Ge}_{30}\) were higher than \(\hbox {C}_{30}\) 5.19, 4.63 and 4.91 eV. \(V_{\mathrm{cell}}\) of K-halogen-, Na-halogen- and \(\hbox {Li-halogen-Ge}_{30}\) are higher than \(\hbox {C}_{30}\) 0.39, 0.36 and 0.32 V. \(\hbox {G}_{\mathrm{ad}}\) of 2, 3 and 4 halogens (F, Br and Cl) on \(\hbox {Ge}_{30}\) are higher than \(\hbox {C}_{30}\) ca 5.12, 3.29 and 4.64 eV, respectively. Finally, the \(\hbox {F-Ge}_{29}\) with high performance and \(V_{\mathrm{cell}}\) was proposed as anode electrode of potassium ion battery.




Similar content being viewed by others
References
Slater M D, Kim D, Lee E and Johnson C S 2013 Adv. Funct. Mater.23 947
Gao W, Haratipour P, Kahkha M R R and Tahvili A 2018 Ultrason. Sonochem.44 152
Mostavi A, Kabir M and Ozevin D 2017 App. Phys. Lett.111 201905
Hu P, Wang X and Wang T 2016 Adv. Sci.3 1600112
Beheshtian J and Peyghan A A 2012 Struct. Chem.23 1567
Salari A A 2017 Inorg. Chim. Acta456 18
Baris O, Malcioglu P and Erkoc S 2005 J. Mol. Graphics Modell.23 367
Bagheri Z and Peyghan A A 2013 Comp. Theor. Chem.1008 20
Peyghan A A, Rastegar S F and Hadipour N L 2014 Phys. Lett. A378 2184
Beheshtian J, Peyghan A A and Noei M 2013 Sens. Actuators B: Chem.181 829
Peyghan A A, Soltani A and Pahlevani A A 2013 Appl. Surf. Sci.270 25
Shi S, Ouyang C, Lei M and Tang W 2007 J. Power Sources171 908
Ahmadi A, Beheshtian J and Kamfiroozi M 2012 J. Mol. Model.18 1729
Shi S, Gao J, Liu Y, Zhao Y, Wu Q and Ju W 2015 Chin. Phys. B25 018212
Soltani A, Ahmadi Peyghan A and Bagheri Z 2013 Physica E48 176
Peyghan A A, Baei M T and Moghimi M 2012 Comp. Theor. Chem.997 63
Beheshtian J, Kamfiroozi M and Bagheri Z 2012 Chin. J. Chem. Phys.25 60
Chowdhury C H, Karmakar S H and Datta A 2016 ACS Energy Lett.1 253
Karmakar S H, Chowdhury C H and Datta A 2016 J. Phys. Chem. C120 14522
Luo W, Shen F, Bommier C, Zhu H, Ji X and Hu L 2016 Acc. Chem. Res.49 231
Tsuneda T, Song J W, Suzuki S and Hirao K 2010 J. Chem. Phys.133 174101
Kar R, Song J W and Hirao K 2013 J. Comput. Chem.34 958
Hosseini J, Rastgou A and Moradi R 2017 J. Mol. Liq.225 913
Najafi M 2017 Can. J. Chem.95 687
Nejati K, Hosseinian A, Bekhradnia A and Vessally E 2017 J. Mol. Graph. Mod.74 1
Hosseinian A, Soleimani S, Arshadi S, Vessally E and Edjlali L 2017 Phys. Lett. A381 2010
Andzelm J and Kolmel C 1995 J. Chem. Phys.103 9312
Gan L H and Zhao J Q 2009 Physica E41 1249
Schmidt M W, Windus T L, Dupuis M and Montgomery J A 1993 J. Comput. Chem.14 1347
Boys S F and Bernardi F 1970 Mol. Phys.19 553
Baghban A, Sasanipour J, Alizad M and Vafaee M 2017 Chem. Eng. Res. Des.126 67
Razavi R, Hosseini S M A and Ranjbar M 2014 Iran J. Chem. Chem. Eng.33 29
Razavi R, Kardani M, Ghanbari A, Lariche M and Baghban A 2018 Petrol. Sci. Technol.36 807
Parsaee Z, Karachi N and Razavi R 2018 Ultrason. Sonochem.47 36
Zahedifar M, Razavi R and Sheibani H 2016 J. Mol. Struct.1125 730
Karachi N, Hosseini M, Parsaee Z and Razavi R 2018 J. Photochem. Photobiol.364 344
Bie R J, Siddiqui M K, Razavi R, Taherkhani M and Najafi M 2018 Acta Chim. Slov.65 303
Sharifian S, Harasek M and Haddadi B 2016 Chem. Prod. Process. Model.11 67
Sharifian S and Harasek M 2015 Chem. Eng. Trans.45 409
Sharifian S, Asasian Kolur N and Harasek M 2019 Energy Sources1 11
Afshar A, Hosseini S and Behzadfar E 2014 Sci. Iran D Comput. Sci. Eng. Electr. Eng.21 2107
Hosseini S A, Gorjian M, Rasouli L and Shirali S 2015 Biosci. Biotech. Res. Asia12 141
Ebrahimi A, Hosseini S A and Rahim F 2014 Cent. Eur. J. Immunol.39 400
Rahim F, Allahmoradi H, Salari F, Shahjahani M, Fard A D, Hosseini S A et al 2013 Int. J. Hematol. Oncol. Stem. Cell Res.7 41
Acknowledgements
We acknowledge 2019 Shanxi Provincial Higher Education Technology Innovation Project: Study on Regulation and Modification of Thermal Synthesis System of LiFeP04/C Composites.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mi, Z., Zhu, K., Ding, L. et al. Investigation of potentials of \(\hbox {C}_{30}\) and \(\hbox {Ge}_{30}\) as anode in metal-ion batteries. Bull Mater Sci 43, 76 (2020). https://doi.org/10.1007/s12034-020-2048-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-020-2048-1