Abstract
An intensified and scalable continuous flow process is presented for the hydroxylation of enolizable tertiary ketones. The procedure relies on molecular oxygen, metal-free conditions and a low toxicity solvent (DMSO). The reaction is optimized on the microfluidic scale with a model ketone substrate (isobutyrophenone) and next extended to a small library of structurally diverse enolizable ketones. High conversion and selectivity are achieved under extremely short residence time. A DFT computational study provides insights on the mechanism and selectivity on various substrates. The scalability of the hydroxylation step is next assessed in a commercial pilot scale continuous flow SiC reactor, hence providing up to 12.5 kg per day of industrially relevant α-ketols with applications ranging from Type I radical photoinitiators to intermediates for the preparation of active pharmaceutical ingredients.

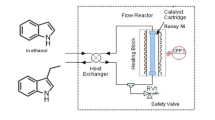
Details of the Corning® Advanced-Flow™ G1 SiC reactor (Courtesy of Corning®) utilized for the intensified continuous flow hydroxylation of tertiary ketones with molecular oxygen.










Similar content being viewed by others
References
Stevens CL, Elliott RD, Winch BL (1963). J Am Chem Soc 85:1464–1470
Stevens CL, Thuillier A, Daniher FA (1965). J Org Chem 30:2962–2966
Stevens CL, Klundt IL, Munk ME, Pillai MD (1965). J Org Chem 30:2967–2972
Stevens CL, Hanson HT, Taylor KG (1966). J Am Chem Soc 88:2769–2774
Stevens CL, Ash AB, Thuillier A, Amin JH, Balys A, Dennis WE, Dickerson JP, Glinski RP, Hanson HT, Pillai MD, Stoddard JW (1966). J Org Chem 31:2593–2601
Stevens CL, Thuillier A, Taylor KG, Daniher FA, Dickerson JP, Hanson HT, Nielsen NA, Tikotkar NA, Weier RM (1966). J Org Chem 31:2601–2607
Stevens CL, Glenn FE, Pillai PM (1973). J Am Chem Soc 95:6301–6308
Paquette LA, Hofferberth JE (2004) The α-Hydroxy ketone (α-Ketol) and related rearrangements in organic reactions. John Wiley & Sons, Inc., Hoboken
Rao HSP, Vijjapu S (2015). Tetrahedron 71:8391–8406
Kassin VEH, Gérardy R, Toupy T, Collin D, Salvadeo E, Toussaint F, Van Hecke K, Monbaliu JCM (2019). Green Chem 21:2952–2966
Uddin MJ, Wilson AJ, Crews BC et al (2019). ACS Omega 4:9251–9261
Steindl J, Koch T, Moszner N, Gorsche C (2017). Macromolecules 50:7448–7457
McGilvray KL, Decan MR, Wang D, Scaiano JC (2006). J Am Chem Soc 128:15980–15981
Marin ML, McGilvray KL, Scaiano JC (2008). J Am Chem Soc 130:16572–16584
Wasserman HH, Lipshutz BH (1975). Tetrahedron Lett 21:1731–1734
Huang JQ, Nairoukh Z, Marek I (2018). Eur J Org Chem 2018:614–618
Liang Y, Wu K, Song S, Li X, Huang X, Jiao N (2015). Org Lett 17:876–879
Wei WT, Zhu WM, Shao Q et al (2018). ACS Sustain Chem Eng 6:8029–8033
Chuang GJ, Wang W, Lee E, Ritter T (2011). J Am Chem Soc 133:1760–1762
Xu S, Wang G, Xu F et al (2018). J Nat Prod 81:1055–1059
Liang YF, Jiao N (2014). Angew Chem Int Ed 53:548–552
Chaudhari MB, Sutar Y, Malpathak S et al (2017). Org Lett 19:3628–3631
Sim SBD, Wang M, Zhao Y (2015). ACS Catal 5:3609–3612
Rahman MT, Nishino H (2003). Org Lett 5:2887–2890
Krabbe SW, Do DT, Johnson JS (2012). Org Lett 14:5932–5935
Giarrusso J, Do DT, Johnson JS (2017). Org Lett 19:3107–3110
Bisht GS, Chaudhari MB, Gupte VS, Gnanaprakasam B (2017). ACS Omega 2:8234–8252
Gandhi H, O’Reilly K, Gupta MK et al (2017). RSC Adv 7:19506–19556
Liu CH, Wang Z, Xiao LY et al (2018). Org Lett 20:4862–4866
Riahi A, Muzart J, Abe M, Hoffmann N (2013). New J Chem 37:2245–2249
Lian M, Li Z, Du J et al (2010). Eur J Org Chem:6525–6530
Yang F, Zhao J, Tang X et al (2017). Org Lett 19:448–451
Tang X-F, Zhao J-N, Wu Y-F et al (2019). Org Biomol Chem 17:7938–7942
Rubottom GM, Gruber JM (1978). J Org Chem 43:1599–1602
Basdevant B, Legault CY (2015). J Org Chem 80:6897–6902
Hone CA, Kappe CO (2019). Top Curr Chem 377:2
Gérardy R, Emmanuel N, Toupy T, Kassin VEH, Tshibalonza NN, Schmitz M, Monbaliu JCM (2018). Eur J Org Chem:2301–2351
Emmanuel N, Mendoza C, Winter M, Horn C, Vizza A, Dreesen L, Heinrichs B, Monbaliu JCM (2017). Org Process Res Dev 21:1435–1438
Richardson WH, Hodge VF, Stiggall DL et al (1974). J Am Chem Soc 96:6652–6657
Sawaki Y, Ogata Y (1975). J Am Chem Soc 97:6983–6989
Sawaki Y, Ogata Y (1976). J Org Chem 98:7324–7327
Eustis S, El-Sayed MA (2006). Chem Soc Rev 35:209–217
Daniel MC, Astruc D (2004). Chem Rev 104:293–346
Saha K, Agasti SS, Kim A, Li X, Rotello VM (2012). Chem Rev 112:2739–2779
Zhou W, Gao X, Liu D, Chen X (2015) 115:10575–10636
Turkevich J, Stevenson PC, Hillier J (1951). Faraday Soc 11:55–75
Zhao P, Li N, Astruc D (2013). Coord Chem Rev 257:638–665
De Freitas LF, Varca GHC, Batista JGDS, Lugão AB (2018). Nanomaterials 8:939
McGilvray KL, Fasciani C, Bueno-Alejo CJ, Schwartz-Narbonne R, Scaiano JC (2012). Langmuir 28:16148–16155
Scaiano JC, Billone P, Gonzalez CM, Maretti L, Marin ML, McGilvray KL, Yuan N (2009). Pure Appl Chem 81:635–647
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 (Revision D.01). Gaussian Inc., Wallingford CT
Acknowledgments
This work was supported by the F.R.S.-FNRS (Fonds National de la Recherche Scientifique, Belgium), the F.R.I.A.-FNRS (Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture, Belgium) and the University of Liège (Welcome Grant WG-13/03, JCMM). VEHK and TT are F.R.I.A.-FNRS PhD student fellows. Computational resources were provided by the “Consortium des Équipements de Calcul Intensif” (CÉCI), funded by the “Fonds de la Recherche Scientifique de Belgique” (F.R.S.-FNRS) under Grant No. 2.5020.11. François Toussaint worked on the preliminary stage of this project within the frame of his Master thesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
Development of a hydroxylation process for enolizable tertiary ketones that relies on metal-free conditions and molecular oxygen.
Identification of the critical process parameters that affect conversion and selectivity through DFT computations.
Intensification of the hydroxylation conditions under scalable conditions using a commercial pilot scale SiC mesofluidic reactor.
Preparation of industrially relevant α-ketols and illustration of a potential application for the photogeneration of radicals and the preparation of gold nanoparticles.
Electronic supplementary material
ESM 1
(DOCX 10638 kb)
Rights and permissions
About this article
Cite this article
Kassin, VE.H., Toupy, T., Petit, G. et al. Metal-free hydroxylation of tertiary ketones under intensified and scalable continuous flow conditions. J Flow Chem 10, 167–179 (2020). https://doi.org/10.1007/s41981-019-00073-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-019-00073-6