Abstract
Background and aim
Dexamethasone Suppression Test (DST), recommended for Cushing’s Syndrome (CS) diagnosis, explores the pituitary feedback to glucocorticoids. Its diagnostic accuracy could be affected by dexamethasone bioavailability, and therefore, we have developed and validated a dexamethasone threshold after 1-mg DST.
Materials and methods
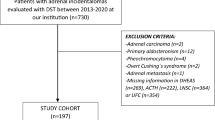
We studied 200 subjects: 125 patients were considered retrospectively and 75 were enrolled prospectively as the validation cohort. Serum dexamethasone, Late Night Salivary Cortisol (LNSC), and Urinary Free Cortisol (UFC) were measured with LC–MS/MS. Normal LNSC and UFC levels were used to exclude CS. The lower 2.5th percentile of dexamethasone distribution in non-CS patients with cortisol ≤ 50 nmol/L after 1-mg DST was used as threshold.
Results
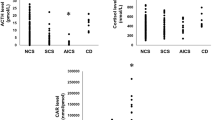
16 patients were CS and 184 non-CS (108 adrenal incidentaloma and 76 excluded CS); 4.5 nmol/L resulted the calculated threshold. Cortisol after 1-mg DST confirmed high sensitivity (100% at 50 nmol/L cut-off) and moderate–low specificity (63%, increased to 91% at 138 nmol/L) to diagnose CS in the whole cohort of patients. We could reduce the number of false-positive results (from 10 to 6 and from 7 to 4 in AI and excluded CS) considering adequate dexamethasone levels. Dexamethasone levels were not affected by hypercortisolism, age, gender, smoke, weight, and creatinine. 6% of non-CS patients did not achieve adequate dexamethasone levels (40% of tests with serum cortisol > 138 nmol/L after 1-mg DST).
Conclusions
We developed and validated the routine dexamethasone measurement during 1-mg DST: it is independent from patient’s clinical presentation, and it should be used to increase the specificity of serum cortisol levels.

Similar content being viewed by others
References
Nieman LK, Biller BMK, Findling JW et al (2008) The diagnosis of cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93(5):1526–1540. https://doi.org/10.1210/jc.2008-0125
Ceccato F, Boscaro M (2016) Cushing’s syndrome: screening and diagnosis. High Blood Press Cardiovasc Prev 23(3):209–215. https://doi.org/10.1007/s40292-016-0153-4
Boscaro M, Arnaldi G (2009) Approach to the patient with possible cushing’s syndrome. J Clin Endocrinol Metab 94(9):3121–3131. https://doi.org/10.1210/jc.2009-0612
Shimon I (2015) Screening for cushing’s syndrome: is it worthwhile? Pituitary 18(2):201–205. https://doi.org/10.1007/s11102-015-0634-9
Ceccato F, Marcelli G, Martino M et al (2018) The diagnostic accuracy of increased late night salivary cortisol for Cushing’s syndrome: a real-life prospective study. J Endocrinol Invest 42(3):327–335. https://doi.org/10.1007/s40618-018-0921-1
Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T (2004) Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res 27(3):193–202. https://doi.org/10.1291/hypres.27.193
Terzolo M, Reimondo G, Chiodini I et al (2012) Screening of Cushing’s syndrome in outpatients with type 2 diabetes: results of a prospective multicentric study in Italy. J Clin Endocrinol Metab 97(10):3467–3475. https://doi.org/10.1210/jc.2012-1323
Terzolo M, Stigliano A, Chiodini I et al (2011) AME position statement on adrenal incidentaloma. Eur J Endocrinol 164(6):851–870. https://doi.org/10.1530/EJE-10-1147
Fassnacht M, Arlt W, Bancos I et al (2016) Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol 175(2):G34. https://doi.org/10.1530/EJE-16-0467
Elamin MB, Murad MH, Mullan R et al (2008) Accuracy of diagnostic tests for Cushing’s syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab 93(5):1553–1562. https://doi.org/10.1210/jc.2008-0139
Pecori Giraldi F, Ambrogio AG, De Martin M, Fatti LM, Scacchi M, Cavagnini F (2007) Specificity of first-line tests for the diagnosis of Cushing’s syndrome: assessment in a large series. J Clin Endocrinol Metab 92(11):4123–4129. https://doi.org/10.1210/jc.2007-0596
Raff H (2009) Utility of salivary cortisol measurements in Cushing’s syndrome and adrenal insufficiency. J Clin Endocrinol Metab 94(10):3647–3655. https://doi.org/10.1210/jc.2009-1166
Nunes M-L, Vattaut S, Corcuff J-B et al (2009) Late-night salivary cortisol for diagnosis of overt and subclinical Cushing’s syndrome in hospitalized and ambulatory patients. J Clin Endocrinol Metab 94(2):456–462. https://doi.org/10.1210/jc.2008-1542
Ceccato F, Barbot M, Zilio M et al (2013) Performance of salivary cortisol in the diagnosis of Cushing’s syndrome, adrenal incidentaloma, and adrenal insufficiency. Eur J Endocrinol 169(1):31–36. https://doi.org/10.1530/EJE-13-0159
Petersenn S, Newell-Price J, Findling JW et al (2014) High variability in baseline urinary free cortisol values in patients with Cushing’s disease. Clin Endocrinol 80(2):261–269. https://doi.org/10.1111/cen.12259
Sandouk Z, Johnston P, Bunch D et al (2018) Variability of late-night salivary cortisol in Cushing disease: a prospective study. J Clin Endocrinol Metab 103(3):983–990. https://doi.org/10.1210/jc.2017-02020
Ceccato F, Antonelli G, Frigo AC et al (2017) First-line screening tests for Cushing’s syndrome in patients with adrenal incidentaloma: the role of urinary free cortisol measured by LC-MS/MS. J Endocrinol Invest 40(7):753–760. https://doi.org/10.1007/s40618-017-0644-8
Morelli V, Scillitani A, Arosio M, Chiodini I (2017) Follow-up of patients with adrenal incidentaloma, in accordance with the European society of endocrinology guidelines: could we be safe? J Endocrinol Invest 40(3):331–333. https://doi.org/10.1007/s40618-016-0558-x
Guthrie S (1991) The impact of dexamethasone pharmacokinetics on the DST: a review. Psychopharmacol Bull 27(4):565–576. https://www.ncbi.nlm.nih.gov/pubmed/1813902
Ueland GÅ, Methlie P, Kellmann R et al (2017) Simultaneous assay of cortisol and dexamethasone improved diagnostic accuracy of the dexamethasone suppression test. Eur J Endocrinol 176(6):705–713. https://doi.org/10.1530/EJE-17-0078
Meikle AW, Lagerquist LG, Tyler FH (1975) Apparently normal pituitary-adrenal suppressibility in Cushing’s syndrome: dexamethasone metabolism and plasma levels. J Lab Clin Med 86(3):472–478. https://www.ncbi.nlm.nih.gov/pubmed/1151162
Meikle AW (1982) Dexamethasone suppression tests: usefulness of simultaneous measurement of plasma cortisol and dexamethasone. Clin Endocrinol 16(4):401–408. https://www.ncbi.nlm.nih.gov/pubmed/7094363
Sasaki Y, Katabami T, Asai S, Fukuda H, Tanaka Y (2017) In the overnight dexamethasone suppression test, 1.0 mg loading is superior to 0.5 mg loading for diagnosing subclinical adrenal Cushing’s syndrome based on plasma dexamethasone levels determined using liquid chromatography-tandem mass spectrometry. Endocr J 64(9):833–842. https://doi.org/10.1507/endocrj.EJ17-0083
Ceccato F, Antonelli G, Barbot M et al (2014) The diagnostic performance of urinary free cortisol is better than the cortisol: cortisone ratio in detecting de novo Cushing’s syndrome: the use of a LC-MS/MS method in routine clinical practice. Eur J Endocrinol 171(1):1–7. https://doi.org/10.1530/EJE-14-0061
Antonelli G, Ceccato F, Artusi C, Marinova M, Plebani M (2015) Salivary cortisol and cortisone by LC-MS/MS: validation, reference intervals and diagnostic accuracy in Cushing’s syndrome. Clin Chim Acta 451:247–251. https://doi.org/10.1016/j.cca.2015.10.004
Valassi E, Swearingen B, Lee H et al (2009) Concomitant medication use can confound interpretation of the combined dexamethasone-corticotropin releasing hormone test in Cushing’s syndrome. J Clin Endocrinol Metab 94(12):4851–4859. https://doi.org/10.1210/jc.2009-1500
A clinical and laboratory standards institute (CLSI), liquid chromatography-mass spectrometry methods; approved guidelines for Clinical Chemistry, available online at https://clsi.org/media/1346/c62a_sample.pdf
Horowitz GL In: Lewis MA (ed) Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved guidelines for Clinical Chemistry, available online https://clsi.org/media/1421/ep28a3c_sample.pdf
Ceccato F, Barbot M, Zilio M et al (2015) Screening tests for cushing’s syndrome: urinary free cortisol role measured by LC-MS/MS. J Clin Endocrinol Metab 100(10):3856–3861. https://doi.org/10.1210/jc.2015-2507
Raff H (2012) Cushing’s syndrome: diagnosis and surveillance using salivary cortisol. Pituitary 15(1):64–70. https://doi.org/10.1007/s11102-011-0333-0
de Graaf AJ, Mulder AL, Krabbe JG (2019) Retrospective analysis of repeated dexamethasone suppression tests—the added value of measuring dexamethasone. Ann Clin Biochem Int J Lab Med 56(6):708–710. https://doi.org/10.1177/0004563219870834
Mayo clinic. https://www.mayocliniclabs.com/test-catalog/Overview/91956
Miller BS, Ignatoski KM, Daignault S et al (2011) A quantitative tool to assess degree of sarcopenia objectively in patients with hypercortisolism. Surgery 150(6):1178–1185. https://doi.org/10.1016/j.surg.2011.09.020
Di Dalmazi G, Vicennati V, Garelli S et al (2014) Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol 2(5):396–405. https://doi.org/10.1016/S2213-8587(13)70211-0
Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J (2014) Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab 99(12):4462–4470. https://doi.org/10.1210/jc.2014-3007
Morelli V, Reimondo G, Giordano R et al (2014) Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab 99(3):827–834. https://doi.org/10.1210/jc.2013-3527
Masserini B, Morelli V, Bergamaschi S et al (2009) The limited role of midnight salivary cortisol levels in the diagnosis of subclinical hypercortisolism in patients with adrenal incidentaloma. Eur J Endocrinol 160(1):87–92. https://doi.org/10.1530/EJE-08-0485
Palmieri S, Morelli V, Polledri E, et al (2013) The role of salivary cortisol measured by liquid chromatography-tandem mass spectrometry in the diagnosis of subclinical hypercortisolism. Eur J Endocrinol 168(3):289. https://www.ncbi.nlm.nih.gov/pubmed/23211572
Ceccato F, Barbot M, Albiger N et al (2017) Daily salivary cortisol and cortisone rhythm in patients with adrenal incidentaloma. Endocrine. https://doi.org/10.1007/s12020-017-1421-3
Fassnacht M, Dekkers OM, Else T et al (2018) European society of endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol 179(4):G1–G46. https://doi.org/10.1530/EJE-18-0608
Trementino L, Appolloni G, Concettoni C, Cardinaletti M, Boscaro M, Arnaldi G (2012) Association of glucocorticoid receptor polymorphism A3669G with decreased risk of developing diabetes in patients with Cushing’s syndrome. Eur J Endocrinol 166(1):35–42. https://doi.org/10.1530/EJE-11-0722
van Rossum EFC, Koper JW, van den Beld AW, et al (2003) Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol 59(5):585–592. https://www.ncbi.nlm.nih.gov/pubmed/14616881
Di Blasio AM, van Rossum EFC, Maestrini S, et al (2003) The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin Endocrinol 59(1):68–74. https://www.ncbi.nlm.nih.gov/pubmed/12807506
Tzanela M, Mantzou E, Saltiki K et al (2012) Clinical and biochemical impact of BCL1 polymorphic genotype of the glucocorticoid receptor gene in patients with adrenal incidentalomas. J Endocrinol Invest 35(4):395–400. https://doi.org/10.3275/7840
Plebani M (2010) The detection and prevention of errors in laboratory medicine. Ann Clin Biochem 47(Pt 2):101–110. https://doi.org/10.1258/acb.2009.009222
Plebani M (2016) Towards a new paradigm in laboratory medicine: the five rights. Clin Chem Lab Med 54(12):1881–1891. https://doi.org/10.1515/cclm-2016-0848
Funding
This study did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest that might be perceived as influencing the impartiality of the reported research.
Ethical approval
The study was performed in accordance with the guidelines in the Declaration of Helsinki, the study was approved by the Ethics Committee of Padova University-Hospital.
Informed consent
All patients gave informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ceccato, F., Artusi, C., Barbot, M. et al. Dexamethasone measurement during low-dose suppression test for suspected hypercortisolism: threshold development with and validation. J Endocrinol Invest 43, 1105–1113 (2020). https://doi.org/10.1007/s40618-020-01197-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01197-6