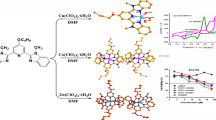
Abstract
Two metal complexes, [Zn(bmbp)Cl2]·H2O (1) and [Co(bmbp)2](ClO4)2·DMF·H2O (2) (bmbp = 4-benzyloxy-2,6-bis(1-methyl-2-benzimidazolyl)pyridine), have been synthesized and characterized. X-ray crystal structure analysis for complex 1 reveals that the Zn(II) atom is penta-coordinated with three nitrogen atoms of the ligand and two chlorine atoms in the coordination sphere, adopting a distorted square pyramidal geometry. For complex 2, the central Co(II) atom is bonded by six nitrogen atoms from two ligands in a distorted octahedral fashion. The two complexes feature 1D chain structures generated by C–H⋯π interactions. The thermal stabilities and spectroscopic properties of the complexes have been studied. Both complexes exhibit inhibition on the growth of the Eca109 cancer cells, but show lower antitumor activity than cisplatin.
Graphic Abstract
Two zinc(II) and cobalt(II) complexes based on 4-benzyloxy-2,6-bis(1-methyl-2-benzimidazolyl)pyridine, which exhibit inhibition on the growth of Eca109 cancer cell, were synthesized and characterized by X-ray single crystal structure analysis.









Similar content being viewed by others
References
Hao JM, Yu BY, Van Hecke K, Cui GH (2015) Cryst Eng Comm 17:2279–2293
Kalinowska-Lis U, Szewczyk EM, Checinska L, Wojciechowski JM, Wolf WM, Ochocki J (2014) Chem Med Chem 9:169–176
Husain A, Rashid M, Shaharyar M, Siddiqui AA, Mishra R (2013) Eur J Med Chem 62:785–798
Chen N, Yang P, He X, Shao M, Li MX (2013) Inorg Chim Acta 405:43–50
Manjunatha MN, Dikundwar AG, Nagasundara KR (2011) Polyhedron 30:1299–1304
Arjmand F, Parveen S, Afzal M, Shahid M (2012) J Photochem Photobiol B 114:15–26
Ou ZB, Lu YH, Lu YM, Chen S, Xiong YH, Zhou XH, Mao ZW, Le XY (2013) J Coord Chem 66:2152–2165
Wu HL, Zhang YH, Zhang JW, Yang ZH, Chen CY, Peng HP, Wang F (2015) Transit Met Chem 40:145–152
Wu HL, Kou F, Jia F, Liu B, Yuan JK, Bai Y (2011) J Photochem Photobiol B 105:190–197
Navarrete-Vázquez G, Cedillo R, Hernández-Campos A, Yépez L, Hernández-Luis F, Valdez J, Morales R, Cortés R, Hernández M, Castillo R (2001) Bioorg Med Chem Lett 11:187–190
Rathore A, Sudhakar R, Ahsan MJ, Ali A, Subbarao N, Jadav SS, Umar S, Shaharyar M (2017) Bioorg Chem 70:107–117
Singla M, Ranjan R, Mahiya K, Mohapatra SC, Ahmad S (2015) New J Chem 39:4316–4327
Rajarajeswari C, Loganathan R, Palaniandavar M, Suresh E, Riyasdeend A, Akbarshae MA (2013) Dalton Trans 42:8347–8363
Hu JY, Guo Y, Zhao JA, Zhang JS (2017) Bioorg Med Chem 25:5733–5742
Ramachandran E, Kalaivani P, Prabhakaran R, Rath NP, Brinda S, Poornima P, Padma VV, Natarajan K (2012) Metallomics 4:218–227
Siddiqi ZA, Kalid M, Kumar S, Shahid M, Novo S (2010) Eur J Med Chem 45:264–269
Bordbar M, Tabatabaee M, Alizadeh-Nouqi M, Mehri-Lighvan Z, Khavasi HR, Yeganeh-Faal A, Fallahian F, Dolati M (2016) J Iran Chem Soc 13:1125–1132
Liu SG, Cao WQ, Yu LL, Zheng WJ, Li LL, Fan CD, Chen TF (2013) Dalton Trans 42:5932–5940
Pyle AM, Barton JK (1990) Prog Inorg Chem Bioinorg Chem 38:413–475
Li GB, Wang SX, Su WY, Pan RK, Liu KD, Liu SG (2014) Russ J Coord Chem 40:764–767
Pan RK, Liu SG, Wang SX, Li GB, Su WY, Huang QW, He YM (2015) Z Anorg Allg Chem 641:627–630
Pan RK, Song JL, Li GB, Lin SQ, Liu SG, Yang GZ (2017) Transit Met Chem 42:253–262
Bashash M, Hislop TG, Shah AM, Le N, Brooks-Wilson A, Bajdik CD (2011) BMC Cancer 11:164
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) CA Cancer J Clin 68:394–424
Pan RK, Li GB, Liu SG, Zhou XP, Yang GZ (2016) Monatsh Chem 147:1189–1196
Sheldrick GM (1997) SHELXS-97, program for the solution of crystal structures. University of Göttingen, Göttingen
Sheldrick GM (1997) SHELXL-97, program for the refinement of crystal structures from diffraction data. University of Göttingen, Göttingen
Spek AL (2009) Acta Cryst D 65:148–155
Addison AW, Rao TN, Reedijk J, Van Rijn J, Verschoor GC (1984) J Chem Soc Dalton Trans 7:1349–1356
Wang D, Liu SX (2007) Polyhedron 26:5469–5476
Zhang LP, Ma JF, Yang J, Liu YY, Wei GH (2009) Cryst Growth Des 9:4660–4673
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 21502085), Special funds for public welfare research and capacity building in Guangdong Province (Nos. 2015A020211038 and 2016A010103042), Research Group of Rare earth resource exploiting and luminescent materials (No. 2017KCXTD022).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10870_2020_824_MOESM2_ESM.doc
Supplementary file2 (DOC 556 kb) CCDC 1401680 and 1449418 contain the supplementary crystallographic data for complexes 1 and 2. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre (CCDC), 12 Union Road, Cam bridge CB2 1EZ, UK.
Rights and permissions
About this article
Cite this article
Pan, RK., Song, JL., Su, WY. et al. Zinc(II) and Cobalt(II) Complexes Derived from 4-Benzyloxy-2,6-bis(1-methyl-2-benzimidazolyl)pyridine: Synthesis, Crystal Structures, Spectroscopic Properties and Antitumour Activities. J Chem Crystallogr 50, 241–248 (2020). https://doi.org/10.1007/s10870-020-00824-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-020-00824-7