Abstract
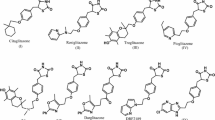
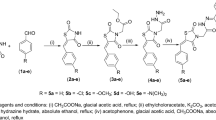
In the present investigation, design, molecular docking simulations, and synthesis of few 2,4-thiazolidinedione for selective modulation of PPAR-gamma are reported. Further evaluation of anti-diabetic activity of few thiazolidine-2,4-diones is assessed using cell line analysis. The structures of the synthesized compounds were confirmed on the basis of FT-IR, 1H-NMR, and mass analyses. Acute toxicity study was done to by using Trypan blue assay and MTT assay of the synthesized compounds. Synthesized compounds were evaluated for their antihyperglycemic effect by glucose absorption assay. The compound code TZD4 showed highest percentage of glucose absorption by 3T3-L1 cells compared with control.










Similar content being viewed by others
References
Wild S, Green RG, Sicree A, R. King H. (2004) Global prevalence of diabetes: estimate for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Maccari R, Ottanà R, Ciurleo R, Rakowitz D, Matuszczak B, Laggner C, Langer T (2008) In vitro evaluation of 5-arylidene-2-thioxo-4-thiazolidinones active as aldose reductase inhibitors. Bioorg. Med. Chem. 16:5840–5847
Janine C, Menciu C, Rakotoarisoa H, Kahn P, Desmurs J (1999) Short synthesis of troglitazone: an anti-diabetic drug for treating drug resistance. Bioorg. Med. Chem. Lett. 9:3439–3440
Prashantha Kumar BR, Nanjan M (2009) Novel glitazones: design, synthesis, glucose uptake and structure activity relationship, med. Chem Res 44(3):1180–1187
Todd L, Suresh T, Mathews, Heidi S (2004) Review: peroxisome proliferator activated receptor-gamma and its role in the development and treatment of diabetes. Exper Diab Res 5:99–109
Youssef J, Mostafa B (2015) Peroxisome proliferator-activated receptors: Features, Functions, and Future. Nuclear Receptor Res 2:1–30
Mark S (2003) Insulin and Hypoglycemic agents. In: Abraham DJ (ed) Berger’s Medicinal Chemistry and Drug Discovery, vol 4. Wiley and Sons Inc, USA, pp 1–37
Singh M, Sharma C (2011) Peroxisome proliferator-activated receptors (PPARs): a target with a broad therapeutic potential for human disease: an overview. Pharmacologyonline 2:58–89
Storr T, Mitchell D, Buglyó P, Thompson K, Yuen V, McNeill H, Orvig C (2003) Vanadyl-thiazolidinedione combination agents for diabetes therapy. Bioconjug. Chem. 14(1):212–221
Gaba M, Gaba P, Singh S, Gupta G (2010) An overview on molecular docking. Int J Drug Develop Res 2:219–231
Prasantha KD, Nanjan MJ (2010) Novel glitazones: design, synthesis, glucose uptake and structure-activity relationships. Bioorg. Med. Chem. Lett. 20:1953–1956
Xuan M, Hong-Xing Z, Mihaly M, Meng C (2011) Molecular docking: a powerful approach for structure-based drug discovery. Current Computer-Aided Drug Design 7:146–157
Das M, Devi G (2015) In vitro cytotoxicity and glucose uptake activity of fruits of Terminalia bellirica in Vero, L-6 and 3T3 cell lines. J. Appl Pharmaceutical Sci 5:92–95
Ernst, R.; Roland, (1955), Organic Syntheses, 3:73
Pandey J, Gilhotra R, Gupta AK (2019) Design, synthesis and evaluation of substituted 5-(2-methoxybenzylidene)-rhodanine ester analogs as aldose reductase inhibitors. Biological Forum Int J 11(1):217–221
Rao P, Kaiser J (2011) Pharmacological evaluation of herbal extracts for their in vitro hypoglycemic activity. Int J Pharm. Bio. Sci 2:12–16
Kadan S, Saad B, Sasson Y, Zaid H (2016) In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chem. 196:1066–1074
Bogna G (2014) Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications—a review. Grygiel-Gorniak Nutritional J 14:13–17
Acknowledgments
The authors would like to acknowledge and give their sincere thanks to the principal and management MET’s Institute of Pharmacy, Nashik India, for providing necessary facilities to carry out this work. The authors would also like to acknowledge the SAIF Chandigarh for undertaking spectroscopic analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3917 kb)
Rights and permissions
About this article
Cite this article
Chhajed, S.S., Shinde, P.E., Kshirsagar, S.J. et al. De-novo design and synthesis of conformationally restricted thiazolidine-2,4-dione analogues: highly selective PPAR-γ agonist in search of anti-diabetic agent. Struct Chem 31, 1375–1385 (2020). https://doi.org/10.1007/s11224-020-01500-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01500-4