Abstract
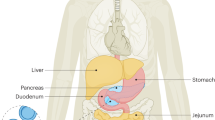
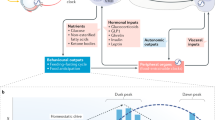
Meal timing and composition are frequently reported in the literature as zeitgebers (that is, time cues) for the circadian system of humans and animal models, albeit secondary to light. Although widely assumed to be true, evidence for food zeitgeber effects specific to humans is notably scarce. Fostering zeitgeber hygiene in the general population as the development and practice of healthy use of zeitgebers could potentially reduce chronobiological strain, which is defined as disruption or misalignment within the circadian system. Such chronobiological strain is associated with modern 24/7 lifestyles (for example, shift work) and several negative health outcomes. Adjustments to meal timing and composition are an attractive strategy to synchronize circadian rhythms and develop zeitgeber hygiene. Thus, clarifying the actual effect of meal timing and composition on the human circadian system is a crucial piece of the human chronobiology puzzle. This Review weighs the evidence from human studies pertaining to the hypothesis that food is a circadian zeitgeber by comparing findings against formal zeitgeber criteria put forward by Jürgen Aschoff in the 1950s.
Key points
-
Fostering zeitgeber hygiene as the practice of healthy use of ‘zeitgebers’ (circadian time cues) could reduce the chronobiological strain (disruption or misalignment within the circadian system) that is associated with modern 24/7 lifestyles and several negative health outcomes.
-
Meal timing and composition are described as ‘zeitgebers’ for humans in the literature, despite a notably paucity of clear, direct evidence from human studies.
-
Only one study has demonstrated that meal timing sufficiently fulfils at least one of the zeitgeber criteria put forward by Jürgen Aschoff in the 1950s.
-
Targeted human research should be prioritized to properly ascertain multifaceted relationships between food intake and human chronobiology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Halberg, F. Physiologic 24-hour periodicity; general and procedural considerations with reference to the adrenal cycle [German]. Int. Z. Vitaminforsch Beih. 10, 225–296 (1959).
Aschoff, J. Die 24-Stunden-Periodik der Maus unter konstanten Umgebungsbedingungen [German]. Die Naturwissenschaften 38, 506–507 (1951).
Aschoff, J. Zeitgeber der tierischen Tagesperiodik [German]. Die Naturwissenschaften 41, 49–56 (1954). An article describing zeitgeber criteria.
Duffy, J. F., Kronauer, R. E. & Czeisler, C. A. Phase-shifting human circadian rhythms: influence of sleep timing, social contact and light exposure. J. Physiol. 495, 289–297 (1996).
Pittendrigh, C. S. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb. Symp. Quant. Biol. 25, 159–184 (1960).
Panda, S. Circadian physiology of metabolism. Science 354, 1008–1015 (2016).
Stenvers, D. J., Scheer, F. A. J. L., Schrauwen, P., la Fleur, S. E. & Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 15, 75–89 (2019).
Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 15, 393–405 (2019).
Russell, G. & Lightman, S. The human stress response. Nat. Rev. Endocrinol. 15, 525–534 (2019).
Ikegami, K., Refetoff, S., Van Cauter, E. & Yoshimura, T. Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 15, 590–600 (2019).
Gabriel, B. M. & Zierath, J. R. Circadian rhythms and exercise — re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 15, 197–206 (2019).
Lewis, P., Foster, R. G. & Erren, T. C. Ticking time bomb? High time for chronobiological research. EMBO Rep. 19, e46073 (2018).
Vetter, C., Fischer, D., Matera, J. L. & Roenneberg, T. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr. Biol. 25, 907–911 (2015).
IARC. Painting, firefighting, and shiftwork. IARC Monogr. Eval. Carcinog. Risks Hum. 98, 9–764 (2010).
West, A. C. et al. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat. Commun. 8, 417 (2017).
IARC. Carcinogenicity of night shift work. Lancet Oncol. 20, 1058–1059 (2019).
Kerenyi, N. A., Pandula, E. & Feuer, G. Why the incidence of cancer is increasing: the role of ‘light pollution’. Med. Hypotheses 33, 75–78 (1990).
Czeisler, C. A. Duration, timing and quality of sleep are each vital for health, performance and safety. Sleep. Health 1, 5–8 (2015).
Czeisler, C. A. Perspective: casting light on sleep deficiency. Nature 497, S13 (2013).
Stevens, R. G. et al. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ. Health Perspect. 115, 1357–1362 (2007).
Erren, T. C. & Reiter, R. J. Revisiting chronodisruption: when the physiological nexus between internal and external times splits in humans. Naturwissenschaften 100, 291–298 (2013).
Erren, T. C. & Lewis, P. Hypothesis: ubiquitous circadian disruption can cause cancer. Eur. J. Epidemiol. 34, 1–4 (2019).
Erren, T. C. & Reiter, R. J. Light hygiene: time to make preventive use of insights—old and new—into the nexus of the drug light, melatonin, clocks, chronodisruption and public health. Med. Hypotheses 73, 537–541 (2009).
Erren, T. C., Falaturi, P. & Reiter, R. J. Research into the chronodisruption-cancer theory: the imperative for causal clarification and the danger of causal reductionism. Neuro. Endocrinol. Lett. 31, 1–3 (2010).
Lewis, P., Korf, H. W., Kuffer, L., Gross, J. V. & Erren, T. C. Exercise time cues (zeitgebers) for human circadian systems can foster health and improve performance: a systematic review. BMJ Open. Sport. Exerc. Med. 4, e000443 (2018).
Richter, C. P. A behavioristic study of the activity of the rat. Comp. Psychol. Monogr. 1, 56 (1922). The first study noting food anticipatory activity in rodents in response to food restriction.
Mistlberger, R. E. et al. Comment on “Differential rescue of light- and food-entrainable circadian rhythms”. Science 322, 675 (2008).
Challet, E. & Mendoza, J. Metabolic and reward feeding synchronises the rhythmic brain. Cell Tissue Res. 341, 1–11 (2010).
Escobar, C. et al. Scheduled meals and scheduled palatable snacks synchronize circadian rhythms: consequences for ingestive behavior. Physiol. Behav. 104, 555–561 (2011).
Mistlberger, R. E. & Skene, D. J. Nonphotic entrainment in humans? J. Biol. Rhythm. 20, 339–352 (2005).
Stephan, F. K. The “other” circadian system: food as a Zeitgeber. J. Biol. Rhythm. 17, 284–292 (2002).
Collado, M. C. et al. Timing of food intake impacts daily rhythms of human salivary microbiota: a randomized, crossover study. FASEB J. 32, 2060–2072 (2018).
Bandin, C. et al. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: a randomized, crossover trial. Int. J. Obes. 39, 828–833 (2015).
Wehrens, S. M. T. et al. Meal timing regulates the human circadian system. Curr. Biol. 27, 1768–1775.e3 (2017). This study demonstrates fulfilment of an Aschoff criterion by meal timing in humans; importantly, effects were observed at the level of circadian genes in a peripheral clock.
Schoeller, D. A., Cella, L. K., Sinha, M. K. & Caro, J. F. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J. Clin. Invest. 100, 1882–1887 (1997).
Sinha, M. K. et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J. Clin. Invest. 97, 1344–1347 (1996).
Cella, L. K., Van Cauter, E. & Schoeller, D. A. Effect of meal timing on diurnal rhythm of human cholesterol synthesis. Am. J. Physiol. 269, E878–E883 (1995).
Fogteloo, A. J., Pijl, H., Roelfsema, F., Frolich, M. & Meinders, A. E. Impact of meal timing and frequency on the twenty-four-hour leptin rhythm. Horm. Res. 62, 71–78 (2004).
Goetz, F. et al. Timing of single daily meal influences relations among human circadian rhythms in urinary cyclic AMP and hemic glucagon, insulin and iron. Experientia 32, 1081–1084 (1976).
Krauchi, K., Cajochen, C., Werth, E. & Wirz-Justice, A. Alteration of internal circadian phase relationships after morning versus evening carbohydrate-rich meals in humans. J. Biol. Rhythm. 17, 364–376 (2002).
Fukuda, T. et al. A randomized, double-blind and placebo-controlled crossover trial on the effect of l-ornithine ingestion on the human circadian clock. Chronobiol. Int. 35, 1445–1455 (2018).
Yasuo, S. et al. l-Serine enhances light-induced circadian phase resetting in mice and humans. J. Nutr. 147, 2347–2355 (2017).
Pivovarova, O. et al. Changes of dietary fat and carbohydrate content alter central and peripheral clock in humans. J. Clin. Endocrinol. Metab. 100, 2291–2302 (2015).
Bergendahl, M., Vance, M. L., Iranmanesh, A., Thorner, M. O. & Veldhuis, J. D. Fasting as a metabolic stress paradigm selectively amplifies cortisol secretory burst mass and delays the time of maximal nyctohemeral cortisol concentrations in healthy men. J. Clin. Endocrinol. Metab. 81, 692–699 (1996).
Cugini, P. et al. Effects of a mild and prolonged restriction in sodium or food intake on the circadian rhythm of aldosterone and related variables. Chronobiol. Int. 4, 245–250 (1987).
Trotti, R., Rondanelli, M., Cuzzoni, G., Ferrari, E. & d’Eril, G. M. Circadian temporal organization of lipidic fractions in elderly people. Entrainment to the dietary schedule. Aging Clin. Exp. Res. 14, 94–99 (2002).
Bogdan, A., Bouchareb, B. & Touitou, Y. Ramadan fasting alters endocrine and neuroendocrine circadian patterns. Meal-time as a synchronizer in humans? Life Sci. 68, 1607–1615 (2001).
Iraki, L. et al. Ramadan diet restrictions modify the circadian time structure in humans. A study on plasma gastrin, insulin, glucose, and calcium and on gastric pH. J. Clin. Endocrinol. Metab. 82, 1261–1273 (1997).
Nakade, M., Takeuchi, H., Taniwaki, N., Noji, T. & Harada, T. An integrated effect of protein intake at breakfast and morning exposure to sunlight on the circadian typology in Japanese infants aged 2–6 years. J. Physiol. Anthropol. 28, 239–245 (2009).
Harada, T., Hirotani, M., Maeda, M., Nomura, H. & Takeuchi, H. Correlation between breakfast tryptophan content and morning-evening in Japanese infants and students aged 0–15 years. J. Physiol. Anthropol. 26, 201–207 (2007).
Cubero, J. et al. The circadian rhythm of tryptophan in breast milk affects the rhythms of 6-sulfatoxymelatonin and sleep in newborn. Neuro Endocrinol. Lett. 26, 657–661 (2005).
Almeneessier, A. S. & BaHammam, A. S. How does diurnal intermittent fasting impact sleep, daytime sleepiness, and markers of the biological clock? Current insights. Nat. Sci. Sleep. 10, 439–452 (2018).
Skene, D. J. et al. Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc. Natl Acad. Sci. USA 115, 7825–7830 (2018).
Skene, D. J. Optimization of light and melatonin to phase-shift human circadian rhythms. J. Neuroendocrinol. 15, 438–441 (2003).
Kennaway, D. J. et al. Phase delay of the rhythm of 6-sulphatoxy melatonin excretion by artificial light. J. Pineal Res. 4, 315–320 (1987).
Grant, C. L. et al. Timing of food intake during simulated night shift impacts glucose metabolism: a controlled study. Chronobiol. Int. 34, 1003–1013 (2017).
Srour, B. et al. Circadian nutritional behaviours and cancer risk: new insights from the NutriNet-sante prospective cohort study: disclaimers. Int. J. Cancer 143, 2369–2379 (2018).
Kogevinas, M. et al. Effect of mistimed eating patterns on breast and prostate cancer risk (MCC-Spain study). Int. J. Cancer 143, 2380–2389 (2018).
Ekhart, D. et al. Dynamics of core body temperature cycles in long-term measurements under real life conditions in women. Chronobiol. Int. 35, 8–23 (2018).
Foster, R. G. et al. Circadian photoreception in the retinally degenerate mouse (rd/rd). J. Comp. Physiol. A 169, 39–50 (1991).
Freedman, M. S. et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284, 502–504 (1999).
Lucas, R. J., Freedman, M. S., Munoz, M., Garcia-Fernandez, J. M. & Foster, R. G. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 284, 505–507 (1999).
Soni, B. G., Philp, A. R., Foster, R. G. & Knox, B. E. Novel retinal photoreceptors. Nature 394, 27–28 (1998).
Hattar, S., Liao, H. W., Takao, M., Berson, D. M. & Yau, K. W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070 (2002).
Sekaran, S., Foster, R. G., Lucas, R. J. & Hankins, M. W. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr. Biol. 13, 1290–1298 (2003).
Provencio, I., Jiang, G., De Grip, W. J., Hayes, W. P. & Rollag, M. D. Melanopsin: an opsin in melanophores, brain, and eye. Proc. Natl Acad. Sci. USA 95, 340–345 (1998).
Kalsbeek, A. et al. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythm. 21, 458–469 (2006).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Misalignment
-
Circadian rhythms are out of sync either with each other or with the external environment; for example, Aschoff criterion 2: upon Zeitgeber reversal, the circadian rhythm is in a transient period of misalignment with regard to the Zeitgeber.
- Internal time
-
The phase of entrained circadian rhythm (for example, dim-light melatonin onset).
- External time
-
Phase of the Zeitgeber rhythm (for example, dawn or dusk).
- Zeitgeber hygiene
-
The practice of synergistic use of Zeitgebers to potentially reduce the chronobiological strain (disruption or misalignment within the circadian system) that is associated with modern 24/7 lifestyles, conflicting Zeitgeber information and several negative health outcomes.
- Masking
-
A direct change in a circadian rhythm caused by another factor, such that it hides the true responsible endogenous component; for example, in humans, pulses of light can inhibit melatonin secretion even if the circadian component is in the acrophase.
- Acrophase
-
The time period during which a rhythm peaks (for example, the crest of a sine wave).
- Mesor
-
The mean of circadian rhythm measurements computed using a cosine function and based on distribution of measurements across cycles.
Rights and permissions
About this article
Cite this article
Lewis, P., Oster, H., Korf, H.W. et al. Food as a circadian time cue — evidence from human studies. Nat Rev Endocrinol 16, 213–223 (2020). https://doi.org/10.1038/s41574-020-0318-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-020-0318-z
This article is cited by
-
The effects of meal patterns on liver steatosis, fibrosis, and biochemical factors in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial
Journal of Diabetes & Metabolic Disorders (2024)
-
Six-hour time-restricted feeding inhibits lung cancer progression and reshapes circadian metabolism
BMC Medicine (2023)
-
Meal timing of dietary total antioxidant capacity and its association with all-cause, CVD and cancer mortality: the US national health and nutrition examination survey, 1999–2018
International Journal of Behavioral Nutrition and Physical Activity (2023)
-
Adjusting phosphate feeding regimen according to daily rhythm increases eggshell quality via enhancing medullary bone remodeling in laying hens
Journal of Animal Science and Biotechnology (2023)
-
Association of meal timing with body composition and cardiometabolic risk factors in young adults
European Journal of Nutrition (2023)