Abstract
The pathogenesis of DNA mismatch repair (MMR)-deficient endometrial carcinoma (EC) is driven by inactivating methylation or less frequently mutation of an MMR gene (MLH1, PMS2, MSH2, or MSH6). This study evaluated the prognostic and clinicopathologic differences between methylation-linked and nonmethylated MMR-deficient endometrioid ECs. We performed MMR immunohistochemistry and methylation-specific multiplex ligation-dependent probe amplification, and classified 682 unselected endometrioid ECs as MMR proficient (MMRp, n = 438) and MMR deficient (MMRd, n = 244), with the latter subcategorized as methylated (MMRd Met) and nonmethylated tumors. Loss of MMR protein expression was detected in 35.8% of the tumors as follows: MLH1 + PMS2 in 29.8%, PMS2 in 0.9%, MSH2 + MSH6 in 1.3%, MSH6 in 2.8%, and multiple abnormalities in 0.9%. Of the 244 MMRd cases, 76% were methylation-linked. MMR deficiency was associated with older age, high grade of differentiation (G3), advanced stage (II–IV), larger tumor size, abundant tumor-infiltrating lymphocytes, PD-L1 positivity in immune cells and combined positive score, wild-type p53, negative L1CAM, ARID1A loss, and type of adjuvant therapy. MMRd-Met phenotype correlated with older age and larger tumor size, and predicted diminished disease-specific survival in the whole cohort. In the MMRd subgroup, univariate analysis demonstrated an association between disease-specific survival and disease stage II–IV, high grade (G3), deep myometrial invasion, lymphovascular invasion, ER negativity, and L1CAM positivity. In conclusion, MMR methylation profile correlates with clinicopathologic characteristics of endometrioid EC, and MMRd-Met phenotype predicts lower disease-specific survival. MMR deficiency, but not MLH1 methylation status, correlates with T-cell inflammation and PD-L1 expression.
Similar content being viewed by others
Introduction
Endometrial carcinoma (EC) is a heterogeneous disease that on a molecular basis can be divided into four subgroups with distinct pathogenesis and prognosis: polymerase ε (POLE) ultramutated, microsatellite instability (MSI) hypermutated, copy-number-low, and copy-number-high tumors [1]. In view of this disease heterogeneity, the development of personalized medicine relies on subclass-specific studies on relevant biomarkers and potential treatment strategies.
Defective DNA mismatch repair (MMRd), leading to instability of microsatellites and high frequency of mutation, is present in 20–40% of ECs [2]. Loss of MMR function results from a biallelic inactivation of the key MMR genes: MLH1, PMS2, MSH2, or MSH6. Sporadic disease is more common, and as regards EC, it generally arises from silencing of MLH1 due to promoter hypermethylation [3, 4]. In some cases, MLH1 methylation reflects a more global promoter hypermethylation pattern (CpG island methylator phenotype, CIMP) [5]. Sporadic tumorigenesis may also be driven by biallelic somatic mutations of the MMR genes [6, 7]. A deleterious germline mutation of one MMR allele defines Lynch syndrome (LS). In a separate and rare mechanism, deletion of the gene coding for epithelial cell adhesion molecule (EPCAM, TACSTD1), leads to constitutional methylation and epigenetic silencing of the adjacent gene, MSH2 [8]. In LS, cancerogenesis is triggered by a “second hit” that inactivates the wild-type allele of the affected MMR gene. Lifetime cancer risk in female patients with LS is 30–43% for colorectal carcinoma (CRC) and 40–60% for EC [9,10,11]. EC represents the sentinel cancer in 35–50% of female LS patients, and it has been estimated that LS accounts for 2–3% of all the ECs [12,13,14]. Hereditary cancer risk is not uniform for all MMR proteins, e.g., female carries of MSH6 germline mutations have a higher risk of EC compared with MLH1 mutation carriers, and their risk for EC is higher than the risk for CRC [15].
Clinical LS screening strategies based on age and family history fail to identify a significant proportion (>40%) of patients with an LS-associated EC [16, 17]. MSI analysis and MMR immunohistochemistry may be used for tumor-based LS screening. These methods identify defective MMR in tumors, but they do not differentiate sporadic defects from LS, which requires germline mutation analysis. In LS-associated tumors, MLH1 promoter methylation is rare, and MLH1 methylation may be used as a surrogate marker for sporadic disease in the screening process [18,19,20].
In CRC, MSI phenotype is considered a positive prognostic factor. Various studies on CRC also suggest that MSI may predict resistance to 5-fluorouracil chemotherapy [21, 22]. The prognostic significance of MMR deficiency in EC has been outlined by the TCGA, but therapeutic implications are unclear. Further, the impact of MLH1 methylation status on prognosis and treatment response needs to be clarified [23,24,25,26]. We classified unselected endometrioid ECs according to MMR protein expression and MLH1 methylation status, and compared MMR subgroups as regards prognosis and the distribution of established clinicopathological risk factors along with various potential molecular biomarkers: estrogen receptor (ER) alpha, progesterone receptor (PR), p53, L1 cell adhesion molecule (L1CAM), AT-rich interactive domain 1A (ARID1A), and an immunotherapy target molecule, programmed death ligand 1 (PD-L1).
Methods
The study was approved by the Institutional Review Board and the National Supervisory Authority for Welfare and Health. A tissue microarray (TMA) was constructed on 842 primary tumor samples from patients who underwent primary surgical treatment for stage I–IV EC at the Department of Obstetrics and Gynecology, Helsinki University Hospital between 2007 and 2012 [27]. The TMA included 745 ECs of the endometrioid subtype. Clinicopathologic data were abstracted from institutional medical and pathology records. Stage was determined according to the FIGO guidelines revised in 2009 [28]. The cutoff for age as a risk factor was based on the finding that age >65 years is an independent poor prognostic factor in endometrial cancer [29]. Disease-specific survival times were calculated as time from surgery to death from EC, and overall survival as time from surgery to death from any cause. Cause of death was mainly based on medical records. Missing data were complemented from death certificates derived from Statistics Finland.
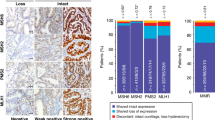
The following monoclonal antibodies were used for chromogenic immunohistochemistry on multicore TMA slides: MLH1 (ES05, Dako), MSH2 (G219-1129, BD Biosciences), MSH6 (EPR3945, Abcam), PMS2 (EPR3947, Epitomics), ERa (SP1, Roche/Ventana), PR (16, Novocastra), p53 (DO-7, Dako), ARID1A (HPA005456, Sigma-Aldrich), and L1CAM (SIG-3911, Covance, clone 14.10). TMA slides were scanned with the 3-dimensional Histech Pannoramic 250 Flash II scanner by Fimmic Oy (Helsinki, Finland). Slide images were managed and analyzed with WebMicroscope Software (Fimmic Oy). Virtual slides were scored by a pathologist blinded to clinical data (AP). A second investigator (RB) examined equivocal cases and a consensus was reached. Mismatch repair protein status was considered deficient (MMR-D) when we observed a complete loss of nuclear expression in carcinoma cells of one or more MMR proteins (MLH1, MSH2, MSH6, and PMS2) detected by immunohistochemistry. MMR proteins form heterodimer complexes (MLH1/PMS2 and MSH2/MSH6), and only MLH1 and MSH2 are stable without their dimer partners. Hence, tumors showing loss of MLH1 and PMS2 or MSH2 and MSH6 on immunohistochemistry, were considered MLH1 and MSH2 defective, respectively. Tumors showing isolated loss of PMS2 or MSH6 in IHC were considered to present inactivation of the homonymous gene. We adopted a 10% cutoff for ER/PR positivity based on common guidelines for breast cancer and a previous study on EC [30]. Aberrant p53 staining (p53 abn) was defined as strong and diffuse nuclear staining or completely negative (“null”) staining in carcinoma cells. Weak and heterogeneous staining was classified as wild-type expression. AT-rich interaction domain 1A (ARID1A) staining was classified negative when tumoral cells presented diffuse or clonal-type loss of nuclear expression. As indicated by a previous mutational study, the heterogeneous “checkerboard” pattern of staining was considered positive [31]. Stromal and inflammatory cells served as internal control for MMR, p53, and ARID1A stainings. L1CAM expression was scored as reported earlier, with ≥10% of membranous staining considered positive [27]. Samples with scarce carcinoma cells or completely negative staining of the internal control (when applicable) were discarded. Representative images of chromogenic IHC are depicted in Fig. 1.
The fluorescent multiplex immunohistochemistry was carried out as reported previously [32]. PD-L1 expression was defined as partial or complete membranous staining in carcinoma cells, and membranous and/or cytoplasmic staining in immune cells (ICs, i.e., CD3-positive T lymphocytes and CD163-positive macrophages within tumor nests and/or adjacent supporting stroma). The percentage of PD-L1-positive carcinoma cells and ICs was estimated separately and in combination. To calculate the combined positive score (CPS), we divided the total number of PD-L1-positive cells (carcinoma cells, lymphocytes, and macrophages) by the number of viable carcinoma cells, multiplied by 100 (www.agilent.com/cs/library/usermanuals/public/29219_pd-l1-ihc-22C3-pharmdx-gastric-interpretation-manual_us.pdf). Cutoff for positive PD-L1 expression was set at ≥1% for all the scorings. The quantity of CD3-positive tumor-infiltrating T lymphocytes (TILs) was visually estimated on multiplex IHC slides, and was semiquantitatively scored as scarce, moderate, or abundant. For statistical analyses tumors with abundant TILs were considered TIL positive.
For DNA extraction, representative areas of formalin-fixed paraffin-embedded tumor sections were macrodissected as identified by pathologist assessment. DNA was extracted by proteinase K/phenol–chloroform method. POLE exonuclease domain mutation screening of hot spots in exon 9, exon 13, and exon 14 was performed by direct sequencing as described before [32]. Polymerase chain reaction products were sequenced on an ABI3730xl Automatic DNA Sequencer at the Institute for Molecular Medicine Finland, Helsinki. Sequence graphs were analyzed both manually and with Mutation Surveyor (Softgenetics, State College, PA).
Methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) was performed on MLH1-deficient tumors to evaluate MLH1 promoter methylation levels in Deng promoter regions C and D. We used the SALSA MMR MS-MLPA Kit ME011 (MRC-Holland) on 250 ng of DNA from each sample. All MS-MLPA reactions, analyses, and calculations of methylation dosage ratios were done according to the manufacturer’s instructions. MS-MLPA products were separated by capillary electrophoresis (on ABI 3730 Automatic DNA sequencer, Applied Biosystems), and analyzed using GeneMapper 5.0 genotyping software (Applied Biosystems). To calculate the methylation ratio, each peak height from HhaI-digested tumor DNA was divided by its corresponding peak height from the undigested tumor DNA. To compensate for differences in PCR efficiency of the individual samples, each peak height (digested and undigested) was normalized, dividing the probe amplification product by the average value of the control probes without a HhaI enzyme site. According to the manufacturer’s recommendations, the hypermethylation threshold is defined as the mean methylation dosage ratio in reference samples (from healthy patients) plus 2 standard deviations. Since our reference samples did not present methylation in the above-mentioned regions, the technical threshold of 0.15 was used as a cutoff [33]. Tumors with a methylation ratio >0.15 (corresponding to 15% of methylated DNA) in region C and/or region D were considered hypermethylated.
Other variables selected for statistical analyses were FIGO stage, age, grade of differentiation, depth of myometrial invasion, lymphovascular invasion (LVI), tumor size, peritoneal cytology, quantity of TILs, expression of PD-L1 (tumor cells, ICs, and CPS), ER, PR, p53, L1CAM, ARID1A, and type of adjuvant therapy. Chi-squared test and Fisher exact test (two-sided) were used for comparison of categorical variables. Survival curves were calculated by the Kaplan–Meier method. A log-rank test was used to test for survival differences. Simple and multivariable analyses for prognostic factors were conducted by the Cox proportional hazard model. Risk factors that were identified as potential predictors in unadjusted analysis were included in the multivariable model. Statistical significance was set at P < 0.05. Data were analyzed using Statistical Package for Social Sciences version 25 software (IBM Corp., Armonk, NY).
Results
Pertinent clinicopathologic characteristics of the study cohort are illustrated in Table 1. MMR immunohistochemistry provided conclusive results for 683/745 cases of endometrioid EC (Fig. 2). Conforming to the original TCGA algorithm, we discarded from the MMRd group 1 tumor that was known to harbor concomitant MLH1 methylation and POLE mutation [1]. Loss of MMR protein expression was observed in 35.8% of the tumors as follows: MLH1 + PMS2 loss in 203 (29.8%), isolated PMS2 loss in six (0.9%), MSH2 + MSH6 loss in nine (1.3%), and isolated MSH6 loss in 19 (2.8%). Concomitant loss of MLH1 + PMS2 and MSH6 was present in seven (1.0%) tumors. Due to uncertain classification, we discarded these seven cases with multiple abnormalities from the methylation status-based analyses.
MS-MLPA was successfully carried out on 157/203 MLH1-/PMS2-negative tumor samples. Cases with unsuccessful methylation analysis (46/203, 22.7%) due to unsuccessful DNA extraction or low-quality DNA, were excluded from the methylation-based analyses. Methylation states of Deng regions C and D were mostly congruent: 132 of 157 samples were methylated, and 14 unmethylated in both regions (P < 0.0001). Methylation of only one of the regions was observed in eleven (7.0%) tumors: six in region C and five in region D. Four of the five cases with isolated hypermethylation in region D, displayed near-cutoff levels of methylation in region C. In total, hypermethylation (methylation ratio >0.15) was observed in 91.1% of the MLH1-deficient tumors. For the purposes of this study, the cases with conclusive IHC and methylation results were divided into subgroups: MMR-proficient tumors (MMRp, n = 438) and MMR-deficient tumors (MMRd, n = 244). MMRd cases with conclusive methylation results were further subclassified as MLH1-methylated (MMRd Met, n = 143) and -nonmethylated (presumably mutated) tumors (MMRd NonMet, n = 48). Considering the methylation rate in this cohort, an estimated 76% of all the MMRd cases and 27% of all the 682 samples presented methylation-linked MMR deficiency. Of all the samples, an estimated 7.6% were MMRd and nonmethylated (presumably mutated).
Proportions of clinicopathologic risk factors and biomarker expression in the molecular subgroups are shown in Table 2. MMR deficiency was associated with older age, high grade of differentiation (G3), advanced stage (II–IV), larger tumor size, abundant TILs, PD-L1 positivity in ICs and CPS, wild-type p53, negative L1CAM, ARID1A loss, and type of adjuvant therapy. Within the MMRd cases, methylation correlated significantly with older age and larger tumor size.
Median follow-up time was 81.3 months (range 1–136) in the whole cohort and 75.7 months (range 2–136) in the MMRd cohort. Disease-specific mortality occurred in 94 (13.8%) of all the 682 patients, including 47/244 (19.3%) of MMRd cases and 47/438 (10.7%) of MMRp cases. Five-year disease-specific survival and overall survival rates were 83.2 and 71.3% for the patients with a MMRd-Met tumor, and 91.7 and 83.3% for the patients with a MMR-NonMet tumor. In the Kaplan–Meier survival analysis based on MMR and methylation status, MMRd-Met phenotype predicted lower disease-specific survival in the whole cohort (P = 0.007, Fig. 3). The negative effect of methylation status retained its significance after controlling for confounders (Table 3). Kaplan–Meier survival analyses were also performed within subgroups of patients receiving radiotherapy, chemotherapy, or both. In these treatment-specific subgroup analyses, we found no statistically significant correlation between MMR phenotype and survival (data not shown). In the simple proportional hazards model performed exclusively on MMRd cases, disease-specific survival was associated with FIGO stage, grade 3 of differentiation, deep myometrial invasion, LVI, peritoneal cytology, tumor diameter, ER negativity, L1CAM positivity, and type of adjuvant therapy, but not methylation (Table 4). Multivariable analysis confirmed the independent effect of LVI (Table 4).
Discussion
EC is a pathogenetically heterogeneous group of sporadic and hereditary tumors. We classified unselected endometrioid ECs according to their MMR IHC profile and MLH1 methylation status in order to explore clinicopathologic differences between MMR subclasses of EC.
The prevalence of specific MMR protein losses was similar to that reported by Watkins et al. [34]. Compared to other studies, the relative frequency of MLH1 loss was high and the frequency of MSH2 loss was low, possibly reflecting geographical differences in the prevalence of various mutations [34,35,36]. In the group of MLH1-deficient cases, 91.1% exhibited hypermethylation, which is in line with previous studies (74–93%) [37, 38]. Methylation of the promoter area is not always complete, and the results of various methylation studies may vary according to the specific promoter regions that have been analyzed [39]. Further, partial methylation of the MLH1 promoter does not invariably lead to gene silencing [39, 40]. To increase specificity of the methylation analysis, we investigated MLH1 methylation exclusively in promoter regions that are confirmedly associated with MLH1 gene silencing [41, 42].
MMRd has been found to correlate with several negative prognostic factors of EC including advanced stage, high grade, LVI, and myometrial invasion, findings corroborated by the present study [24, 26, 43, 44]. However, in the pre-TCGA studies, where dichotomous comparisons between MMRd and MMRp subgroups were made, MMRd was generally not associated with poor survival [45]. In the TCGA study with four molecular subgroups, MMRd along with the copy-number-low ECs, presented an intermediate prognosis compared with the indolent POLE mutated and the aggressive copy-number-high ECs [1]. As regards the prognostic significance of methylation status in EC, we found an association between the MLH1-methylated phenotype and lower disease-specific survival as compared with MMRp EC. Similarly, in the study by Cosgrove et al., poorer recurrence-free survival was observed in the subgroup of methylated MMRd EC as compared with the nonmethylated phenotypes [26]. Shikama et al. reported poor overall survival in patients with sporadic EC compared with nonmethylated (presumed LS) MMRd tumors [25]. It is noteworthy that when measuring overall survival (as opposed to disease-specific survival), differences between presumed hereditary and sporadic disease may reflect the divergent age distribution in these groups. In our study this effect was mitigated by the adoption of disease-specific survival as the measure of prognosis.
Along with the traditional clinicopathological factors and methylation status, we investigated the expression of various biomarkers in the MMR subgroups of EC. MMRd carcinomas rarely displayed aberrant p53 or L1CAM positivity, both considered molecular markers of aggressive EC [1, 27, 46, 47]. Mutation of ARID1A, a chromatin-remodeling protein, is frequent in endometrioid ECs [48]. Compared with MMRp, we observed ARID1A protein loss more frequently in MMRd tumors, with a particularly high frequency in MMRd-Met tumors. The expression of ER, PR, p53, L1CAM, or ARID1A did not correlate independently with survival in the MMRd subgroup.
Based on abounding data on CRC, detection of MMR deficiency may predict the efficacy of adjuvant therapies. The relationship between MMR phenotype and response to adjuvant treatment has not been fully established for EC [24, 25, 49]. Preclinical studies have provided evidence suggesting that impaired MMR confers resistance to certain chemotherapeutic agents, including platinum-based compounds, which are commonly used in the treatment of gynecological cancers [50]. By contrast, defective MMR has predicted better response to adjuvant radiotherapy in EC [51, 52]. Our study could not demonstrate a significant correlation between MMR status and response to adjuvant therapies. Subclass-specific studies on treatment response generally suffer from low power, which could be increased by meta-analyses conducted on larger amounts of subclass data. Interestingly, defective MMR appears to predict favorable response to antiPD1/PD-L1 immunotherapy [53]. In fact, MMRd carcinomas characteristically present higher T-cell counts and more frequent PD-L1 positivity (T-cell inflamed PD-L1 positive phenotype) [32, 54]. It is not known whether these findings are universal across various subgroups of MMRd carcinomas (sporadic vs. hereditary, methylated vs. mutated). Previous studies suggest that ECs with mutation-associated MMR deficiency present higher quantities of TILs compared with ECs with epigenetic MMRd, but data on PD-L1 expression are contrasting [54,55,56]. In our cohort MMRd tumors presented higher quantities of TILs and more frequent PD-L1 positivity on ICs and CPS compared with MMRp tumors, but we could not demonstrate significant immunological differences between methylation-based phenotypes of MMRd ECs.
As an advantage, our single center study provides sufficiently long follow-up times and accurate information on causes of death. We chose to investigate MMR deficiency with immunohistochemistry instead of MSI analysis because of the additional information it offers on the specific MMR proteins. Four-antibody immunohistochemistry and MSI analysis predict mutations with comparable sensitivities. Rare cases with mutations producing nonfunctional proteins with retained immunohistochemical antigenicity, may be misinterpreted by IHC [57]. By contrast, MSH6 mutations are more frequently detected by IHC [58, 59]. We strictly reported MMR deficiency only for cases showing adequate staining of the internal control. TMA-based methodology allows to perform numerous immunohistochemical stainings on a vast number of tumor samples. On the other hand, uneven distribution of protein expression may lead to false negativity in a TMA study. Previous studies have shown that TMAs with 2–3 core biopsies per tumor adequately represent the tumor phenotype, even for heterogeneous antigens such as ER, PR, p53, and Her-2 [60, 61]. To increase sensitivity, we included four cores from each tumor in our TMA. We previously demonstrated a high concordance between the same TMA and whole section stainings of L1CAM, a highly heterogeneous antigen [27]. In addition, our prevalence of MMR deficiency was at the upper end of the expected values. We thus consider our TMA adequately representative of the MMR status in our study cohort. Lack of germline data may lead to erroneous classification into sporadic and hereditary disease. In fact, a deleterious germline mutation is detected in only half of the EC patients classified as “probable LS” (MMR deficiency not associated with MLH1 methylation) [62]. Possible pathogenetic mechanisms of this “Lynch-like syndrome” include currently unidentified germline mutations, somatic cell mosaicism, or biallelic somatic mutation [7, 34, 35, 63]. Acknowledging this unsolved genetic scene, we based our study on protein expression and methylation phenotypes without further assumptions about sporadic or hereditary origin of the disease.
In conclusion, MMR protein expression and MLH1 methylation profiles define distinctive phenotypes that correlate with prognostic factors and immunologic features of EC. Methylation-linked MMRd phenotype predicts poor survival in endometrioid EC. Further studies are necessary to investigate the predictive value of MMR subclasses for both traditional adjuvant therapy and immunotherapy.
References
Cancer Genome Atlas Research Network, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73.
MacDonald ND, Salvesen HB, Ryan A, Iversen OE, Akslen LA, Jacobs IJ. Frequency and prognostic impact of microsatellite instability in a large population-based study of endometrial carcinomas. Cancer Res. 2000;60:1750–2.
Simpkins SB, Bocker T, Swisher EM, Mutch DG, Gersell DJ, Kovatich AJ, et al. MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Hum Mol Genet. 1999;8:661–6.
Esteller M, Levine R, Baylin SB, Ellenson LH, Herman JG. MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene. 1998;17:2413–7.
Whitcomb BP, Mutch DG, Herzog TJ, Rader JS, Gibb RK, Goodfellow PJ. Frequent HOXA11 and THBS2 promoter methylation, and a methylator phenotype in endometrial adenocarcinoma. Clin Cancer Res. 2003;9:2277–87.
Haraldsdottir S, Hampel H, Tomsic J, Frankel WL, Pearlman R, de la Chapelle A, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147:1308.e1–16.
Mensenkamp AR, Vogelaar IP, van Zelst–Stams WAG, Goossens M, Ouchene H, Hendriks–Cornelissen SJB, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology. 2014;146:643.e8–6.
Kovacs ME, Papp J, Szentirmay Z, Otto S, Olah E. Deletions removing the last exon of TACSTD1 constitute a distinct class of mutations predisposing to Lynch syndrome. Hum Mutat. 2009;30:197–203.
Aarnio M, Mecklin JP, Aaltonen LA, Nystrom-Lahti M, Jarvinen HJ. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer. 1995;64:430–3.
Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105–10.
Stoffel E, Mukherjee B, Raymond VM, Tayob N, Kastrinos F, Sparr J, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621–7.
Moller P, Seppala T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017;66:464–72.
Lu KH, Dinh M, Kohlmann W, Watson P, Green J, Syngal S, et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol. 2005;105:569–74.
Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7.
Moller P, Seppala TT, Bernstein I, Holinski-Feder E, Sala P, Gareth Evans D, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the prospective Lynch syndrome database. Gut. 2018;67:1306–16.
Mills AM, Liou S, Ford JM, Berek JS, Pai RK, Longacre TA. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am J Surg Pathol. 2014;38:1501–9.
Kahn RM, Gordhandas S, Maddy BP, Baltich Nelson B, Askin G, Christos PJ, et al. Universal endometrial cancer tumor typing: how much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125:3172–83.
Bettstetter M, Dechant S, Ruemmele P, Grabowski M, Keller G, Holinski-Feder E, et al. Distinction of hereditary nonpolyposis colorectal cancer and sporadic microsatellite-unstable colorectal cancer through quantification of MLH1 methylation by real-time PCR. Clin Cancer Res. 2007;13:3221–8.
Joensuu EI, Abdel-Rahman WM, Ollikainen M, Ruosaari S, Knuutila S, Peltomaki P. Epigenetic signatures of familial cancer are characteristic of tumor type and family category. Cancer Res. 2008;68:4597–605.
Buchanan DD, Tan YY, Walsh MD, Clendenning M, Metcalf AM, Ferguson K, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol. 2014;32:90–100.
Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46:2788–98.
Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–18.
Zighelboim I, Goodfellow PJ, Gao F, Gibb RK, Powell MA, Rader JS, et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007;25:2042–8.
McMeekin DS, Tritchler DL, Cohn DE, Mutch DG, Lankes HA, Geller MA, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34:3062–8.
Shikama A, Minaguchi T, Matsumoto K, Akiyama-Abe A, Nakamura Y, Michikami H, et al. Clinicopathologic implications of DNA mismatch repair status in endometrial carcinomas. Gynecol Oncol. 2016;140:226–33.
Cosgrove CM, Cohn DE, Hampel H, Frankel WL, Jones D, McElroy JP, et al. Epigenetic silencing of MLH1 in endometrial cancers is associated with larger tumor volume, increased rate of lymph node positivity and reduced recurrence-free survival. Gynecol Oncol. 2017;146:588–95.
Pasanen A, Tuomi T, Isola J, Staff S, Butzow R, Loukovaara M. L1 cell adhesion molecule as a predictor of disease-specific survival and patterns of relapse in endometrial cancer. Int J Gynecol Cancer. 2016;26:1465–71.
Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4.
Benedetti Panici P, Basile S, Salerno MG, Di Donato V, Marchetti C, Perniola G, et al. Secondary analyses from a randomized clinical trial: age as the key prognostic factor in endometrial carcinoma. Am J Obstet Gynecol. 2014;210:363.e1–e10.
Jongen V, Briet J, de Jong R, ten Hoor K, Boezen M, van der Zee A, et al. Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynecol Oncol. 2009;112:537–42.
Guan B, Mao TL, Panuganti PK, Kuhn E, Kurman RJ, Maeda D, et al. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011;35:625–32.
Pasanen A, Ahvenainen T, Pellinen T, Vahteristo P, Loukovaara M, Butzow R. PD-L1 expression in endometrial carcinoma cells and intratumoral immune cells: differences across histologic and TCGA-based molecular subgroups. Am J Surg Pathol. 2019;44:174–81.
Porkka N, Lahtinen L, Ahtiainen M, Böhm JP, Kuopio T, Eldfors S, et al. Epidemiological, clinical and molecular characterization of Lynch-like syndrome: a population-based study. Int J Cancer. 2019;145:87–98.
Watkins JC, Yang EJ, Muto MG, Feltmate CM, Berkowitz RS, Horowitz NS, et al. Universal screening for mismatch-repair deficiency in endometrial cancers to identify patients with Lynch syndrome and Lynch-like syndrome. Int J Gynecol Pathol. 2017;36:115–27.
Najdawi F, Crook A, Maidens J, McEvoy C, Fellowes A, Pickett J, et al. Lessons learnt from implementation of a Lynch syndrome screening program for patients with gynaecological malignancy. Pathology. 2017;49:457–64.
Haruma T, Nagasaka T, Nakamura K, Haraga J, Nyuya A, Nishida T, et al. Clinical impact of endometrial cancer stratified by genetic mutational profiles, POLE mutation, and microsatellite instability. PLoS ONE. 2018;13:e0195655.
Bruegl AS, Djordjevic B, Urbauer DL, Westin SN, Soliman PT, Lu KH, et al. Utility of MLH1 methylation analysis in the clinical evaluation of Lynch syndrome in women with endometrial cancer. Curr Pharm Des. 2014;20:1655–63.
Goodfellow PJ, Billingsley CC, Lankes HA, Ali S, Cohn DE, Broaddus RJ, et al. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: an NRG Oncology and Gynecologic Oncology Group Study. J Clin Oncol. 2015;33:4301–8.
Capel E, Flejou JF, Hamelin R. Assessment of MLH1 promoter methylation in relation to gene expression requires specific analysis. Oncogene. 2007;26:7596–600.
Deng G, Chen A, Hong J, Chae HS, Kim YS. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res. 1999;59:2029–33.
Deng G, Peng E, Gum J, Terdiman J, Sleisenger M, Kim YS. Methylation of hMLH1 promoter correlates with the gene silencing with a region-specific manner in colorectal cancer. Br J Cancer. 2002;86:574–9.
Yuen ST, Chan TL, Ho JW, Chan AS, Chung LP, Lam PW, et al. Germline, somatic and epigenetic events underlying mismatch repair deficiency in colorectal and HNPCC-related cancers. Oncogene. 2002;21:7585–92.
Black D, Soslow RA, Levine DA, Tornos C, Chen SC, Hummer AJ, et al. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol. 2006;24:1745–53.
An HJ, Kim KI, Kim JY, Shim JY, Kang H, Kim TH, et al. Microsatellite instability in endometrioid type endometrial adenocarcinoma is associated with poor prognostic indicators. Am J Surg Pathol. 2007;31:846–53.
Ruiz I, Martin-Arruti M, Lopez-Lopez E, Garcia-Orad A. Lack of association between deficient mismatch repair expression and outcome in endometrial carcinomas of the endometrioid type. Gynecol Oncol. 2014;134:20–3.
Zeimet AG, Reimer D, Huszar M, Winterhoff B, Puistola U, Azim SA, et al. L1CAM in early-stage type I endometrial cancer: results of a large multicenter evaluation. J Natl Cancer Inst. 2013;105:1142–50.
Bosse T, Nout RA, Stelloo E, Dreef E, Nijman HW, Jürgenliemk-Schulz IM, et al. L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: pooled PORTEC trial results. Eur J Cancer. 2014;50:2602–10.
McConechy MK, Ding J, Cheang MC, Wiegand K, Senz J, Tone A, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012;228:20–30.
Kim SR, Pina A, Albert A, McAlpine J, Wolber R, Blake Gilks C, et al. Does MMR status in endometrial cancer influence response to adjuvant therapy? Gynecol Oncol. 2018;151:76–81.
Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4:1–6.
Resnick KE, Frankel WL, Morrison CD, Fowler JM, Copeland LJ, Stephens J, et al. Mismatch repair status and outcomes after adjuvant therapy in patients with surgically staged endometrial cancer. Gynecol Oncol. 2010;117:234–8.
Reijnen C, Kusters-Vandevelde HVN, Prinsen CF, Massuger LFAG, Snijders MPML, Kommoss S, et al. Mismatch repair deficiency as a predictive marker for response to adjuvant radiotherapy in endometrial cancer. Gynecol Oncol. 2019;154:124–30.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl J Med. 2015;372:2509–20.
Pakish JB, Zhang Q, Chen Z, Liang H, Chisholm GB, Yuan Y, et al. Immune microenvironment in microsatellite-instable endometrial cancers: hereditary or sporadic origin matters. Clin Cancer Res. 2017;23:4473–81.
Chavez JA, Wei L, Suarez AA, Parwani AV, Li Z. Clinicopathologic characteristics, tumor infiltrating lymphocytes and programed cell death ligand-1 expression in 162 endometrial carcinomas with deficient mismatch repair function. Int J Gynecol Cancer. 2019;29:113–8.
Sloan EA, Ring KL, Willis BC, Modesitt SC, Mills AM. PD-L1 expression in mismatch repair-deficient endometrial carcinomas, including Lynch syndrome-associated and MLH1 promoter hypermethylated tumors. Am J Surg Pathol. 2017;41:326–33.
Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: part II. The utility of microsatellite instability testing. J Mol Diagn. 2008;10:301–7.
Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10:293–300.
Pinol V, Castells A, Andreu M, Castellvi-Bel S, Alenda C, Llor X, et al. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA. 2005;293:1986–94.
Fons G, Hasibuan SM, van der Velden J, ten Kate FJ. Validation of tissue microarray technology in endometrioid cancer of the endometrium. J Clin Pathol. 2007;60:500–3.
Camp RL, Charette LA, Rimm DL. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000;80:1943–9.
Buchanan DD, Rosty C, Clendenning M, Spurdle AB, Win AK. Clinical problems of colorectal cancer and endometrial cancer cases with unknown cause of tumor mismatch repair deficiency (suspected Lynch syndrome). Appl Clin Genet. 2014;7:183–93.
Xicola RM, Clark JR, Carroll T, Alvikas J, Marwaha P, Regan MR, et al. Implication of DNA repair genes in Lynch-like syndrome. Fam Cancer. 2019;18:331–42.
Acknowledgements
We thank Prof. Päivi Peltomäki for sharing her expertise on methylation analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pasanen, A., Loukovaara, M. & Bützow, R. Clinicopathological significance of deficient DNA mismatch repair and MLH1 promoter methylation in endometrioid endometrial carcinoma. Mod Pathol 33, 1443–1452 (2020). https://doi.org/10.1038/s41379-020-0501-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0501-8
This article is cited by
-
Prognostic value of mismatch repair deficiency in patients receiving first-line fluoropyrimidine plus platinum for metastatic, recurrent, or unresectable gastric cancer
Gastric Cancer (2024)
-
Prävention des Endometriumkarzinoms bei Lynch-Syndrom
Die Gynäkologie (2023)
-
Identification and clinical validation of NUSAP1 as a novel prognostic biomarker in ovarian cancer
BMC Cancer (2022)
-
Microsatellite instability as a marker of prognosis: a systematic review and meta-analysis of endometrioid endometrial cancer survival data
Archives of Gynecology and Obstetrics (2022)