Abstract
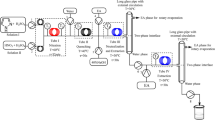
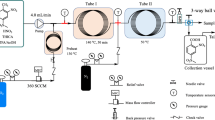
A synthesis process of 5-fluoro-2-nitrobenzotrifluoride was achieved in a continuous-flow millireactor system through the nitration of 3-fluorobenzotrifluoride using the mixed acid as a nitrating agent. The process safety was evaluated based on the temperature rise with the use of Reaction Calorimeter and Differential Scanning Calorimetry. The mass transfer limitation for this nitration process in the millireactor was evaluated. Effects of the composition of the mixed acid, the molar ratio of nitric acid to the substrate, the residence time, and the temperature on the reaction performance in the millireactor were studied in order to obtain optimal operational conditions. A heat transfer assessment was made to discuss the effects of the residence time and the inner diameter on the temperature distribution and the substrate conversion. Compared with the traditional batch reactor, the flow reactor could achieve better control over impurity and higher process efficiency because of its enhanced mass and heat transfer rates under the optimized conditions. This continuous-flow synthesis strategy would be beneficial for the commercial manufacturing of fine chemicals and pharmaceutical intermediates with the involvement of nitration.

.











Similar content being viewed by others
References
Mei H, Han J, Fustero S, Medio-Simon M, Sedgwick DM, Santi C, Ruzziconi R, Soloshonok VA (2019) Fluorine-containing drugs approved by the FDA in 2018. Chem Eur J 25:11797–11819
Fang C, Jing Y, Zong Y, Lin Z (2016) Preparation and characterization of fluorine-containing acrylic latex PSAs using a reactive surfactant. J Fluor Chem 192:113–119
Hauel N, Ceci A, Doods H, Mack J, Priepke H., Walter R., Wiedenmayer D. Boehringer Ingelheim International GMBH. US 2010/0240669 A1
Kulkarni AA (2014) Continuous flow nitration in miniaturized devices. Beilstein J Org Chem 10:405–424
Yasukouchi H, Nishiyama A, Mitsuda M (2018) Safe and efficient Phosgenation reactions in a continuous flow reactor. Org Process Res Dev 22:247–251
Mason BP, Price KE, Steinbacher JL, Bogdan AR, McQuade DT (2007) Greener approaches to organic synthesis using microreactor technology. Chem Rev 107:2300–2318
Newman SG, Jensen KF (2013) The role of flow in green chemistry and engineering. Green Chem 15:1456
Halder R, Lawal A, Damavarapu R (2007) Nitration of toluene in a microreactor. Catal Today 125:74–80
Kulkarni AA, Kalyani VS, Joshi RA, Joshi RR (2009) Continuous flow nitration of Benzaldehyde. Org Process Res Dev 13:999–1002
Kulkarni AA, Nivangune NT, Kalyani VS, Joshi RA, Joshi RR (2008) Continuous flow nitration of salicylic acid. Org Process Res Dev 12:995–1000
Gage JR, Guo X, Tao J, Zheng C (2012) High output continuous nitration. Org Process Res Dev 16:930–933
Wen Z, Jiao F, Yang M, Zhao S, Zhou F, Chen G (2017) Process development and scale-up of the continuous flow nitration of Trifluoromethoxybenzene. Org Process Res Dev 21:1843–1850
Roberge DM, Zimmermann B, Rainone F, Gottsponer M, Eyholzer M, Kockmann N (2008) Microreactor technology and continuous processes in the fine chemical and pharmaceutical industry: is the revolution underway? Org Process Res Dev 12:905–910
Bogdan AR, Dombrowski AW (2019) Emerging trends in flow chemistry and applications to the pharmaceutical industry. J Med Chem 62:6422–6468
Roberge DM, Ducry L, Bieler N, Cretton P, Zimmermann B (2005) Microreactor technology: a revolution for the fine chemical and pharmaceutical industries? Chem Eng Technol 28:318–323
Su Y, Kuijpers K, Hessel V, Noël T (2016) A convenient numbering-up strategy for the scale-up of gas–liquid photoredox catalysis in flow. React Chem Eng 1:73–81
Qiu M, Zha L, Song Y, Xiang L, Su Y (2019) Numbering-up of capillary microreactors for homogeneous processes and its application in free radical polymerization. React Chem Eng 4:351–361
De la Mare PBD, Ridd JH (1959) Aromatic substitution. Academic Press, New York
Brocklehurst CE, Lehmann H, La Vecchia L (2011) Nitration chemistry in continuous flow using fuming nitric acid in a commercially available flow reactor. Org Process Res Dev 15:1447–1453
Su Y, Zhao Y, Jiao F, Chen G, Yuan Q (2011) The intensification of rapid reactions for multiphase systems in a microchannel reactor by packing microparticles. AICHE J 57:1409–1418
Finger G, Reed FH (1944) Aromatic fluorine compounds. I. the synthesis of 2, 5-and 3, 5-Difluorobenzotrifluorides1. J Am Chem Soc 66:1972–1974
Humm AW, Schneider MR (1990) Entwicklung nichtsteroidaler Antiandrogene: 4-Nitro-3-trifluormethyldiphenylamine. Arch Pharm (Weinheim Ger) 323:83–87
Ghaffarzadeh M, Rahbar S (2014) A novel method for synthesis of Flutamide on the bench-scale. J Chem Res 38:200–201
Ducry L, Roberge DM (2005) Controlled autocatalytic nitration of phenol in a microreactor. Angew Chem Int Ed 44:7972–7975
Biswas KG, Das G, Ray S, Basu JK (2015) Mass transfer characteristics of liquid–liquid flow in small diameter conduits. Chem Eng Sci 122:652–661
Ghaini A, Kashid MN, Agar DW (2010) Effective interfacial area for mass transfer in the liquid–liquid slug flow capillary microreactors. Chem Eng Process 49:358–366
Bourne JR (2003) Mixing and the selectivity of chemical reactions. Org Process Res Dev 7:471–508
Maestri F, Copelli S, Rota R, Gigante L, Lunghi A, Cardillo P (2009) Simple procedure for optimal scale-up of fine chemical processes. II. Nitration of 4-Chlorobenzotrifluoride. Ind Eng Chem Res 48:1316–1324
Vassena D, Kogelbauer A, Prins R (2000) Potential routes for the nitration of toluene and nitrotoluene with solid acids. Catal Today 60:275–287
Zhang C, Zhang J, Luo G (2016) Kinetic study and intensification of acetyl Guaiacol nitration with nitric acid-acetic acid system in a microreactor. J Flow Chem 6:309–314
Howell G, James M (1995) Raman spectroscopic study of nitronium ion formation in mixtures of nitric acid, sulfuric acid and water. J. Chem. Soc. Faraday Trans 91:1439–1443
Li G, Pu X, Shang M, Zha L, Su Y (2019) Intensification of liquid–liquid two-phase mass transfer in a capillary microreactor system. AICHE J 65:334–346
Li G, Shang M, Song Y, Su Y (2018) Characterization of liquid–liquid mass transfer performance in a capillary microreactor system. AICHE J 64:1106–1116
Yap SK, Yuan Y, Zheng L, Wong WK, Yan N, Khan SA (2014) Triphasic segmented flow Millireactors for rapid nanoparticle—catalyzed gas-liquid reactions — hydrodynamic studies and reactor modeling. J Flow Chem 4:200–205
Potdar A, Thomassen LCJ, Kuhn S (2019) Structured porous Millireactors for liquid-liquid chemical reactions. Chem Ing Tech 91:592–601
Acknowledgments
We would like to acknowledge financial support from the National Natural Science Foundation of China (No. 21676164) and the Science and Technology Commission of Shanghai Municipality (No. 18520743500). We also thank a safety engineer Mr. Guoxiang Wei from Porton Pharma Solutions, Ltd. for his assistance on the analysis of reaction heat release.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 141 kb)
Rights and permissions
About this article
Cite this article
Chen, P., Shen, C., Qiu, M. et al. Synthesis of 5-fluoro-2-nitrobenzotrifluoride in a continuous-flow millireactor with a safe and efficient protocol. J Flow Chem 10, 207–218 (2020). https://doi.org/10.1007/s41981-019-00068-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-019-00068-3