Abstract
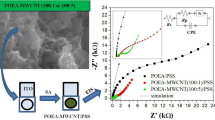
In this work, composites based on polyaniline (PAni) grafted with reduced graphene oxide (rGO) were obtained by the in situ chemical polymerization of aniline with rGO (mass ratio of 1 and 2.5%) dispersed into the monomer solutions. PAni and its PAni-rGO composites were used to prepare self-assembled (SA) films by depositing them onto indium tin oxide (ITO) substrates with alternating layers of polystyrene sulfonate (PSS). The structure and morphology of the materials were characterized by Fourier-transform infrared spectroscopy (FT-IR), RAMAN spectroscopy, and scanning electron microscopy (SEM). The oxidation states of PAni and its PAni-rGO composites were characterized by cyclic voltammetry (CV) and UV-Vis spectroscopy during the SA procedure. The electrochemical behavior of the obtained SA films was also characterized by electrochemical impedance spectroscopy (EIS). The EIS results showed a significant decrease in the polarization resistance (Rp), from 1020 to 302 Ω, for the film with 1% of rGO (PAni-1%rGO/PSS) when compared with its unmodified counterpart (PAni/PSS). The synergic effects observed for the PAni-1%rGO/PSS film showed that controlling the rGO mass ratio plays an important role in the improvement of the charge transfer processes, and that this electrode has potential for electrochemical applications, such as sensors and charge storage devices.






Similar content being viewed by others
References
Cincotto FH, Moraes FC, Spinola Machado SA (2014) Graphene nanosheets and quantum dots: a smart material for electrochemical applications. Chem Eur J 20:4746–4753
Kang X, Wang J, Wu H, Liu J, Aksay IA, Lin Y (2010) A graphene-based electrochemical sensor for sensitive detection of paracetamol. Talanta 81(3):754–759
Wang L, Dong J, Wang Y et al (2016) Novel signal-amplified fenitrothion electrochemical assay, based on glassy carbon electrode modified with dispersed graphene oxide. Sci Rep 6:1–8
Lei W, Si W, Xu Y et al (2014) Conducting polymer composites with graphene for use in chemical sensors and biosensors. Microchim Acta 181:707–722
Silva BVM, Rodriguez BAG, Sales GF et al (2016) An ultrasensitive human cardiac troponin T graphene screen-printed electrode based on electropolymerized-molecularly imprinted conducting polymer. Biosens Bioelectron 77:978–985
Takamura T, Endo K, Fu L et al (2007) Identification of nano-sized holes by TEM in the graphene layer of graphite and the high rate discharge capability of Li-ion battery anodes. Electrochim Acta 53:1055–1061
Jiang M, Yuan Z, Qiao F, Lian Z, Yu F, Dong F, Xing LB (2019) Dimethylsulfoxide-dependent environments for fabricating graphene hydrogels for high-performance supercapacitor. J Nanosci Nanotechnol 19:5755–5761
Xu C, Shi X, Ji A et al (2015) Fabrication and characteristics of reduced graphene oxide produced with different green reductants. PLoS One 10:1–15
Li X, Zhong Q, Zhang X et al (2015) In-situ polymerization of polyaniline on the surface of graphene oxide for high electrochemical capacitance. Thin Solid Films 584:348–352
Zhang J, Yang H, Shen G, Cheng P, Zhang J, Guo S (2010) Reduction of graphene oxide via L-ascorbic acid. Chem Commun 46:1112–1114
Almeida DAL, Couto AB, Ferreira NG (2019) Flexible polyaniline/reduced graphene oxide/carbon fiber composites applied as electrodes for supercapacitors. J Alloys Compd 788:453–460
Li J, Xiao D, Ren Y et al (2019) Bridging of adjacent graphene/polyaniline layers with polyaniline nanofibers for supercapacitor electrode materials. Electrochim Acta 300:193–201
Semwal V, Gupta BD (2019) Highly sensitive surface plasmon resonance based fiber optic pH sensor utilizing rGO-Pani nanocomposite prepared by in situ method. Sensors Actuators B Chem 283:632–642
Xie A, Du J, Zhang J et al (2019) A high-performance nonenzymatic urea sensor based on graphene-NiO-polyaniline. J Electrochem Soc 166:B456–B463
Zhao N, Ma Z, Song H et al (2018) Polyaniline/reduced graphene oxide-modified carbon fiber brush anode for high-performance microbial fuel cells. Int J Hydrog Energy 43:17867–17872
Li Z, Yang S, Song Y et al (2019) In-situ modified titanium suboxides with polyaniline/graphene as anode to enhance biovoltage production of microbial fuel cell. Int J Hydrog Energy 44:6862–6870
Sofyan N, Nugraha RA, Ridhova A et al (2018) Characteristics of PANi/rGO Nanocomposite as protective coating and catalyst in dye-sensitized solar cell counter electrode deposited on AISI 1086 steel substrate. Int J Eng 31:1741–1748
Nemade K, Dudhe P, Tekade P (2018) Enhancement of photovoltaic performance of polyaniline/graphene composite-based dye-sensitized solar cells by adding TiO2 nanoparticles. Solid State Sci 83:99–106
Wang L, Lu X, Lei S, Song Y (2014) Graphene-based polyaniline nanocomposites: preparation, properties and applications. J Mater Chem A 2:4491–4509
Coskun E, Zaragoza-Contreras EA, Salavagione HJ (2012) Synthesis of sulfonated graphene/polyaniline composites with improved electroactivity. Carbon 50:2235–2243
Detsri E, Dubas ST (2013) Interfacial polymerization of polyaniline and its layer-by-layer assembly into polyelectrolytes multilayer thin-films. J Appl Polym Sci 128:558–565
Cena CR, Malmonge LF, Malmonge JA (2016) Layer-by-layer thin films of polyaniline alternated with natural rubber and their potential application as a chemical sensor. J Polym Res 24:1–7
Zhang D, Wang D, Li P et al (2018) Facile fabrication of high-performance QCM humidity sensor based on layer-by-layer self-assembled polyaniline/graphene oxide nanocomposite film. Sensors Actuators B Chem 255:1869–1877
Roteta M, Fernández-Martínez R, Mejuto M, Rucandio I (2016) Preparation of graphene thin films for radioactive samples. Appl Radiat Isot 109:217–221
Rosa T, Aroeira GJR, Parreira LS et al (2015) Self-assembled films based on polyaniline/multiwalled carbon nanotubes composites and sulphonated polystyrene deposited onto ITO substrates. Synth Met 210:186–191
Khanra P, Kuila T, Bae SH et al (2012) Electrochemically exfoliated graphene using 9-anthracene carboxylic acid for supercapacitor application. J Mater Chem 22:24403–24410
Gao R, Hu N, Yang Z et al (2013) Paper-like graphene-Ag composite films with enhanced mechanical and electrical properties. Nanoscale Res Lett 8:32
Ossonon BD, Belanger D (2017) Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheets. RSC Adv 7:27224–27234
He D, Peng Z, Gong W et al (2015) Mechanism of a green graphene oxide reduction with reusable potassium carbonate. RSC Adv 5:11966–11972
Guezzout Z, Doufnoune R, Haddaoui N (2017) Effect of graphene oxide on the properties of compatibilized polypropylene/ethylene-propylene-rubber blend. J Polym Res 24:129
Trchova M, Stejskal J (2011) Polyaniline: the infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl Chem 83:1803–1817
Perumbilavil S, Sankar P, Rose TP, Philip R (2015) White light Z-scan measurements of ultrafast optical nonlinearity in reduced graphene oxide nanosheets in the 400-700 nm region. Appl Phys Lett 107:1–5
Muzyka R, Drewniak S, Pustelny T et al (2018) Characterization of graphite oxide and reduced graphene oxide obtained from different graphite precursors and oxidized by different methods using Raman spectroscopy. Materials 11:1050
Meyer JC, Geim AK, Katsnelson MI, Novoselov KS, Booth TJ, Roth S (2007) The structure of suspended graphene sheets. Nature 446(7131):60–63
Yin H, Shi X, He C et al (2019) Stone-Wales graphene: a two-dimensional carbon semimetal with magic stability. Phys Rev B 99:041405
Boyer MI, Quillard S, Rebourt E et al (1998) Vibrational analysis of polyaniline: a model compound approach. J Phys Chem B 102:7382–7392
Devi M, Kumar A (2020) Surface modification of reduced graphene oxide-polyaniline nanotubes nanocomposites for improved supercapacitor electrodes. Polym Compos 41:653–667
Liu J, Duan Y, Song L et al (2019) Heterogeneous nucleation promoting formation and enhancing microwave absorption properties in hierarchical sandwich-like polyaniline/graphene oxide induced by mechanical agitation. Compos Sci Technol 182:107780
Zhang Y, Liu J, Zhang Y et al (2017) Facile synthesis of hierarchical nanocomposites of aligned polyaniline nanorods on reduced graphene oxide nanosheets for microwave absorbing materials. RSC Adv 7:54031–54038
Song E, Choi J-W (2013) Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials 3(3):498–523
Harish C, SreeHarsha VS, Santhosh C et al (2012) Synthesis of polyaniline/graphene nanocomposites and its optical, electrical and electrochemical properties. Adv Sci Eng Med 4:1–9
Girotto EM, De Paoli MA (1999) Mass transport in intrinsically conducting polymers: importance, techniques and theoretical models. Quim Nova 22:358–368
Hong S-Y, Park S-M (2007) Electrochemistry of conductive polymers 40. Earlier phases of aniline polymerization studied by fourier transform electrochemical impedance spectroscopy. J Phys Chem B 111:9779–9786
Wang Z, Han J-J, Zhang N et al (2019) Synthesis of polyaniline/graphene composite and its application in zinc-rechargeable batteries. J Solid State Electrochem 23:3373–3382
Andre RS, Shimizu FM, Miyazaki CM et al (2017) Hybrid layer-by-layer (LbL) films of polyaniline, graphene oxide and zinc oxide to detect ammonia. Sensors Actuators B Chem 238:795–801
Acknowledgments
The authors thank CAPES for providing the PhD fellowship.
Funding
This study is supported by the São Paulo Research Foundation (Fundação de Amparo à pesquisa do Estado de São Paulo), FAPESP grant number 2017/24742-7, providing financial and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Araújo, G.M., Codognoto, L. & Simões, F.R. Self-assembled electrodes based on polyaniline grafted with reduced graphene oxide and polystyrene sulfonate. J Solid State Electrochem 24, 1857–1866 (2020). https://doi.org/10.1007/s10008-020-04517-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04517-1