Abstract
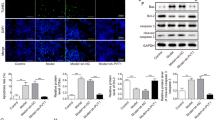
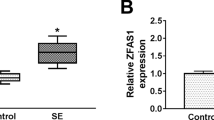
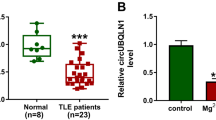
Although many advances have been made in the pathogenesis of epilepsy recently, the pathological mechanisms of epilepsy are still largely unknown. Exploring the pathological mechanisms and developing novel therapeutic strategies for epilepsy are urgently needed. A SD rat model of epilepsy was established with lithium chloride-pilocarpine. Astrocytes were isolated, cultured from 8 to 12 week rats and identified by flow cytometry and immunofluorescence. Immunohistochemical staining was used for MEF2C and NF-κB in paraffin-embedded sections. RT-qPCR and western blot were used to analyze gene expression. ELISA was used to analyze the concentration of IL-6, TNF-α and Cox-2. Cells were transfected with pcDNA-MEFC2, sh-MEFC2, pcDNA-UCA1, sh-UCA1, miR-203 mimic or miR-203 inhibitor. Cell viability was assessed by MTT assay. Dual luciferase assay was used to determine the direct interaction of lncRNA UCA1/miR-203 and miR-203/MEF2C. MEF2C was down-regulated and inhibited NF-κB expression and the secretion of IL-6 and TNF-α in epilepsy. LncRNA UCA1 was also down-regulated in epilepsy. LncRNA UCA1 over-expression increased the expression of MEF2C and its knock-down decreased MEF2C expression. Luciferase activity showed lncRNA UCA1 directly targeted miR-203 and miR-203 directly targeted MEF2C. MiR-203 suppressed the expression of MEF2C, and promoted NF-κB, phosphorylated IκB/IKK and inflammatory effectors, which was reversed by MEF2C knock-down. Moreover, lncRNA UCA1 could increase the expression of MEF2C to inhibit NF-κB, phosphorylated IκB/IKK and inflammatory effectors, which was also reversed by miR-203 mimic transfection. LncRNA UCA1 inhibited the inflammation via regulating miR-203 mediated regulation of MEF2C/NF-κB signaling in epilepsy. Our investigation elucidated novel pathological mechanisms and provided potential therapeutic targets for epilepsy.







Similar content being viewed by others
References
Devinsky O et al (2018) Epilepsy: nature reviews. Dis Primers 4:18024. https://doi.org/10.1038/nrdp.2018.24
Hui Yin, Y., Ahmad, N. & Makmor-Bakry, M. Pathogenesis of epilepsy: challenges in animal models. Iran J Basic Med Sci 16:1119–1132
Rana A, Musto AE (2018) The role of inflammation in the development of epilepsy. J Neuroinflamm 15:144. https://doi.org/10.1186/s12974-018-1192-7
Musto AE, Gjorstrup P, Bazan NG (2011) The omega-3 fatty acid-derived neuroprotectin D1 limits hippocampal hyperexcitability and seizure susceptibility in kindling epileptogenesis. Epilepsia 52:1601–1608. https://doi.org/10.1111/j.1528-1167.2011.03081.x
Musto AE et al (2016) Dysfunctional epileptic neuronal circuits and dysmorphic dendritic spines are mitigated by platelet-activating factor receptor antagonism. Sci Rep 6:30298. https://doi.org/10.1038/srep30298
Borlot F, Whitney R, Cohn RD, Weiss SK (2019) MEF2C-related epilepsy: Delineating the phenotypic spectrum from a novel mutation and literature review. Seizure 67:86–90. https://doi.org/10.1016/j.seizure.2019.03.015
Xu Z et al (2015) Transcription factor MEF2C suppresses endothelial cell inflammation via regulation of NF-kappaB and KLF2. J Cell Physiol 230:1310–1320. https://doi.org/10.1002/jcp.24870
Dhanoa JK, Sethi RS, Verma R, Arora JS, Mukhopadhyay CS (2018) Long non-coding RNA: its evolutionary relics and biological implications in mammals: a review. J Anim Sci Technol 60:25. https://doi.org/10.1186/s40781-018-0183-7
Batista PJ, Chang HY (2013) Long noncoding RNAs: cellular address codes in development and disease. Cell 152:1298–1307. https://doi.org/10.1016/j.cell.2013.02.012
Geng JF et al (2018) LncRNA UCA1 inhibits epilepsy and seizure-induced brain injury by regulating miR-495/Nrf2-ARE signal pathway. Int J Biochem cell Biol 99:133–139. https://doi.org/10.1016/j.biocel.2018.03.021
Lee ST et al (2017) Inhibition of miR-203 reduces spontaneous recurrent seizures in rats. Mol Neurobiol 54:3300–3308. https://doi.org/10.1007/s12035-016-9901-7
Luo W et al (2014) The transient expression of miR-203 and its inhibiting effects on skeletal muscle cell proliferation and differentiation. Cell Death Dis 5:e1347. https://doi.org/10.1038/cddis.2014.289
Gong P et al (2018) LncRNA UCA1 promotes tumor metastasis by inducing miR-203/ZEB2 axis in gastric cancer. Cell Death Dis 9:1158. https://doi.org/10.1038/s41419-018-1170-0
Grolla AA et al (2013) Amyloid-beta and Alzheimer's disease type pathology differentially affects the calcium signalling toolkit in astrocytes from different brain regions. Cell Death Dis 4:e623. https://doi.org/10.1038/cddis.2013.145
Foo LC et al (2011) Development of a method for the purification and culture of rodent astrocytes. Neuron 71:799–811. https://doi.org/10.1016/j.neuron.2011.07.022
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2017) Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol 54:2969–2985. https://doi.org/10.1007/s12035-016-9880-8
Liu YX et al (2012) Exposure to 1950-MHz TD-SCDMA electromagnetic fields affects the apoptosis of astrocytes via caspase-3-dependent pathway. PLoS ONE 7:e42332. https://doi.org/10.1371/journal.pone.0042332
Faure JB et al (2013) A comprehensive behavioral evaluation in the lithium-pilocarpine model in rats: effects of carisbamate administration during status epilepticus. Epilepsia 54:1203–1213. https://doi.org/10.1111/epi.12219
Jang Y et al (2018) Dysregulated long non-coding RNAs in the temporal lobe epilepsy mouse model. Seizure 58:110–119. https://doi.org/10.1016/j.seizure.2018.04.010
Luo ZH et al (2019) Construction and analysis of a dysregulated lncRNA-associated ceRNA network in a rat model of temporal lobe epilepsy. Seizure 69:105–114. https://doi.org/10.1016/j.seizure.2019.04.010
Sun SC (2017) The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol 17:545–558. https://doi.org/10.1038/nri.2017.52
Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G (2012) The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol 22:557–566. https://doi.org/10.1016/j.tcb.2012.08.001
Stafstrom, C. E. & Carmant, L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harbor perspectives in medicine 5, doi:10.1101/cshperspect.a022426 (2015).
Tang F, Hartz AMS, Bauer B (2017) Drug-resistant epilepsy: multiple hypotheses, few answers. Front Neurol 8:301. https://doi.org/10.3389/fneur.2017.00301
Vezzani A, Aronica E, Mazarati A, Pittman QJ (2013) Epilepsy and brain inflammation. Exp Neurol 244:11–21. https://doi.org/10.1016/j.expneurol.2011.09.033
Vezzani A, Granata T (2005) Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46:1724–1743. https://doi.org/10.1111/j.1528-1167.2005.00298.x
Seifert G, Carmignoto G, Steinhauser C (2010) Astrocyte dysfunction in epilepsy. Brain Res Rev 63:212–221. https://doi.org/10.1016/j.brainresrev.2009.10.004
Steinhauser C, Seifert G, Bedner P (2012) Astrocyte dysfunction in temporal lobe epilepsy: K+ channels and gap junction coupling. Glia 60:1192–1202. https://doi.org/10.1002/glia.22313
Shen Y et al (2016) Postnatal activation of TLR4 in astrocytes promotes excitatory synaptogenesis in hippocampal neurons. J Cell Biol 215:719–734. https://doi.org/10.1083/jcb.201605046
Cao Q, Liu X, Yang F, Wang H (2018) CB2R induces a protective response for epileptic seizure via the PI3K 110alpha-AKT signaling pathway. Exp Ther Med 16:4784–4790. https://doi.org/10.3892/etm.2018.6788
Chiavegato A, Zurolo E, Losi G, Aronica E, Carmignoto G (2014) The inflammatory molecules IL-1beta and HMGB1 can rapidly enhance focal seizure generation in a brain slice model of temporal lobe epilepsy. Front Cell Neurosci 8:155. https://doi.org/10.3389/fncel.2014.00155
Sama MA et al (2008) Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. J Biol Chem 283:21953–21964. https://doi.org/10.1074/jbc.M800148200
Robel S et al (2015) Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci 35:3330–3345. https://doi.org/10.1523/JNEUROSCI.1574-14.2015
Bellot-Saez A, Kekesi O, Morley JW, Buskila Y (2017) Astrocytic modulation of neuronal excitability through K(+) spatial buffering. Neurosci Biobehav Rev 77:87–97. https://doi.org/10.1016/j.neubiorev.2017.03.002
Dossi E, Vasile F, Rouach N (2018) Human astrocytes in the diseased brain. Brain Res Bull 136:139–156. https://doi.org/10.1016/j.brainresbull.2017.02.001
Chen X, Gao B, Ponnusamy M, Lin Z, Liu J (2017) MEF2 signaling and human diseases. Oncotarget 8:112152–112165. https://doi.org/10.18632/oncotarget.22899
Shalizi AK, Bonni A (2005) brawn for brains: the role of MEF2 proteins in the developing nervous system. Curr Top Dev Biol 69:239–266. https://doi.org/10.1016/S0070-2153(05)69009-6
Phan D et al (2005) BOP, a regulator of right ventricular heart development, is a direct transcriptional target of MEF2C in the developing heart. Development 132:2669–2678. https://doi.org/10.1242/dev.01849
Verdaguer E et al (2005) Inhibition of the cdk5/MEF2 pathway is involved in the antiapoptotic properties of calpain inhibitors in cerebellar neurons. Br J Pharmacol 145:1103–1111. https://doi.org/10.1038/sj.bjp.0706280
Pon JR, Marra MA (2016) MEF2 transcription factors: developmental regulators and emerging cancer genes. Oncotarget 7:2297–2312. https://doi.org/10.18632/oncotarget.6223
Leifer D et al (1993) MEF2C, a MADS/MEF2-family transcription factor expressed in a laminar distribution in cerebral cortex. Proc Natl Acad Sci USA 90:1546–1550. https://doi.org/10.1073/pnas.90.4.1546
Latchney SE, Jiang Y, Petrik DP, Eisch AJ, Hsieh J (2015) Inducible knockout of Mef2a, -c, and -d from nestin-expressing stem/progenitor cells and their progeny unexpectedly uncouples neurogenesis and dendritogenesis in vivo. FASEB J 29:5059–5071. https://doi.org/10.1096/fj.15-275651
Lee DY et al (2015) Dysregulation of long non-coding RNAs in mouse models of localization-related epilepsy. Biochem Biophys Res Commun 462:433–440. https://doi.org/10.1016/j.bbrc.2015.04.149
Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA (2013) Glia and epilepsy: excitability and inflammation. Trends Neurosci 36:174–184. https://doi.org/10.1016/j.tins.2012.11.008
Jimenez-Mateos EM, Henshall DC (2013) Epilepsy and microRNA. Neuroscience 238:218–229. https://doi.org/10.1016/j.neuroscience.2013.02.027
Primo MN, Bak RO, Schibler B, Mikkelsen JG (2012) Regulation of pro-inflammatory cytokines TNFalpha and IL24 by microRNA-203 in primary keratinocytes. Cytokine 60:741–748. https://doi.org/10.1016/j.cyto.2012.07.031
Wang Y, Dong Q, Gu Y, Groome LJ (2016) Up-regulation of miR-203 expression induces endothelial inflammatory response: Potential role in preeclampsia. Am J Reprod Immunol 76:482–490. https://doi.org/10.1111/aji.12589
Acknowledgements
This work was supported by the grant from the New Xiangya Talent Project of the Third Xiangya Hospital, Central South University, China (JY201721).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Q., Zhao, MW. & Yang, P. LncRNA UCA1 Suppresses the Inflammation Via Modulating miR-203-Mediated Regulation of MEF2C/NF-κB Signaling Pathway in Epilepsy. Neurochem Res 45, 783–795 (2020). https://doi.org/10.1007/s11064-019-02952-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02952-9