Abstract
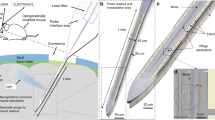
The use of nanophotonics to rapidly and precisely reconfigure light beams for the optical stimulation of neurons in vivo has remained elusive. Here we report the design and fabrication of an implantable silicon-based probe that can switch and route multiple optical beams to stimulate identified sets of neurons across cortical layers and simultaneously record the produced spike patterns. Each switch in the device consists of a silicon nitride waveguide structure that can be rapidly (<20 μs) reconfigured by electrically tuning the phase of light. By using an eight-beam probe, we show in anaesthetized mice that small groups of single neurons can be independently stimulated to produce multineuron spike patterns at sub-millisecond precision. We also show that a probe integrating co-fabricated electrical recording sites can simultaneously optically stimulate and electrically measure deep-brain neural activity. The technology is scalable, and it allows for beam focusing and steering and for structured illumination via beam shaping. The high-bandwidth optical-stimulation capacity of the device might facilitate the probing of the spatiotemporal neural codes underlying behaviour.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, but they are available for research purposes from the corresponding authors on reasonable request.
Code availability
The code packages MClust 3.5 and CellBase R2013a are openly available at http://redishlab.neuroscience.umn.edu/mclust/MClust.html and https://github.com/hangyabalazs/CellBase.
References
Atabaki, A. H. et al. Integrating photonics with silicon nanoelectronics for the next generation of systems on a chip. Nature 556, 349–354 (2018).
Xu, Q., Schmidt, B., Pradhan, S. & Lipson, M. Micrometre-scale silicon electro-optic modulator. Nature 435, 325–327 (2005).
Reed, G. T., Mashanovich, G., Gardes, F. Y. & Thomson, D. J. Silicon optical modulators. Nat. Photon. 4, 518–526 (2010).
Dai, D., Wang, J., Chen, S., Wang, S. & He, S. Monolithically integrated 64-channel silicon hybrid demultiplexer enabling simultaneous wavelength- and mode-division-multiplexing: monolithically integrated 64-channel silicon hybrid demultiplexer. Laser Photon. Rev. 9, 339–344 (2015).
Sun, J., Timurdogan, E., Yaacobi, A., Hosseini, E. S. & Watts, M. R. Large-scale nanophotonic phased array. Nature 493, 195–199 (2013).
Hutchison, D. N. et al. High-resolution aliasing-free optical beam steering. Optica 3, 887 (2016).
Phare, C. T., Shin, M. C., Miller, S. A., Stern, B. & Lipson, M. Silicon optical phased array with high-efficiency beam formation over 180 degree field of view. Preprint at https://arxiv.org/abs/1802.04624 (2018).
Shen, Y. et al. Deep learning with coherent nanophotonic circuits. Nat. Photon. 11, 441–446 (2017).
Harris, N. C. et al. Quantum transport simulations in a programmable nanophotonic processor. Nat. Photon. 11, 447–452 (2017).
Segev, E. et al. Patterned photostimulation via visible-wavelength photonic probes for deep brain optogenetics. Neurophotonics 4, 011002 (2016).
Shim, E., Chen, Y., Masmanidis, S. & Li, M. Multisite silicon neural probes with integrated silicon nitride waveguides and gratings for optogenetic applications. Sci. Rep. 6, 22693 (2016).
Li, B., Lee, K., Masmanidis, S. C. & Li, M. A nanofabricated optoelectronic probe for manipulating and recording neural dynamics. J. Neural Eng. 15, 046008 (2018).
Selvaraja, S. K., Bogaerts, W., Dumon, P., Van Thourhout, D. & Baets, R. Subnanometer linewidth uniformity in silicon nanophotonic waveguide devices using CMOS fabrication technology. IEEE J. Sel. Top. Quantum Electron. 16, 316–324 (2010).
Choy, J. T. et al. Integrated TiO2 resonators for visible photonics. Opt. Lett. 37, 539 (2012).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Du, J., Blanche, T. J., Harrison, R. R., Lester, H. A. & Masmanidis, S. C. Multiplexed, high density electrophysiology with nanofabricated neural probes. PLoS ONE 6, e26204 (2011).
Pisanello, F. et al. Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber. Nat. Neurosci. 20, 1180–1188 (2017).
Wu, F. et al. Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron 88, 1136–1148 (2015).
Kim, T.-I. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Scharf, R. et al. Depth-specific optogenetic control in vivo with a scalable, high-density μLED neural probe. Sci. Rep. 6, 28381 (2016).
Shemesh, O. A. et al. Temporally precise single-cell-resolution optogenetics. Nat. Neurosci. 20, 1796–1806 (2017).
Peron, S. & Svoboda, K. From cudgel to scalpel: toward precise neural control with optogenetics. Nat. Methods 8, 30–34 (2011).
Yang, Y., DeWeese, M. R., Otazu, G. H. & Zador, A. M. Millisecond-scale differences in neural activity in auditory cortex can drive decisions. Nat. Neurosci. 11, 1262–1263 (2008).
deCharms, R. C. & Merzenich, M. M. Primary cortical representation of sounds by the coordination of action-potential timing. Nature 381, 610–613 (1996).
Hahnloser, R. H. R., Kozhevnikov, A. A. & Fee, M. S. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419, 65–70 (2002).
Watts, M. R. et al. Adiabatic thermo-optic Mach–Zehnder switch. Opt. Lett. 38, 733–735 (2013).
Cai, X. et al. Integrated compact optical vortex beam emitters. Science 338, 363–366 (2012).
Ji, N., Freeman, J. & Smith, S. L. Technologies for imaging neural activity in large volumes. Nat. Neurosci. 19, 1154–1164 (2016).
Tanizawa, K. et al. Ultra-compact 32 × 32 strictly-non-blocking Si-wire optical switch with fan-out LGA interposer. Opt. Express 23, 17599 (2015).
Phare, C. T., Daniel Lee, Y.-H., Cardenas, J. & Lipson, M. Graphene electro-optic modulator with 30 GHz bandwidth. Nat. Photon. 9, 511–514 (2015).
Poulton, C. V. et al. Coherent solid-state LIDAR with silicon photonic optical phased arrays. Opt. Lett. 42, 4091 (2017).
Zhang, F. et al. The microbial opsin family of optogenetic tools. Cell 147, 1446–1457 (2011).
Zortman, W. A., Trotter, D. C. & Watts, M. R. Silicon photonics manufacturing. Opt. Express 18, 23598–23607 (2010).
Wagner, H., Brill, S., Kempter, R. & Carr, C. E. Microsecond precision of phase delay in the auditory system of the barn owl. J. Neurophysiol. 94, 1655–1658 (2005).
Garcia-Lazaro, J. A., Belliveau, L. A. C. & Lesica, N. A. Independent population coding of speech with sub-millisecond precision. J. Neurosci. 33, 19362–19372 (2013).
Bale, M. R., Campagner, D., Erskine, A. & Petersen, R. S. Microsecond-scale timing precision in rodent trigeminal primary afferents. J. Neurosci. 35, 5935–5940 (2015).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Hoffman, L. et al. High-density optrode-electrode neural probe using SixNy photonics for in vivo optogenetics. In IEEE International Electron Devices Meeting (ed. Suehle, J.) 29.5.1–29.5.4. (IEEE, 2015).
Zorzos, A. N., Scholvin, J., Boyden, E. S. & Fonstad, C. G. Three-dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Opt. Lett. 37, 4841–4843 (2012).
Zorzos, A. N., Boyden, E. S. & Fonstad, C. G. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Opt. Lett. 35, 4133–4135 (2010).
Schwaerzle, M., Paul, O. & Ruther, P. Compact silicon-based optrode with integrated laser diode chips, SU-8 waveguides and platinum electrodes for optogenetic applications. J. Micromech. Microeng. 27, 065004 (2017).
Ji, X. et al. Ultra-low-loss on-chip resonators with sub-milliwatt parametric oscillation threshold. Optica 4, 619–624 (2017).
Thomson, D. J., Hu, Y., Reed, G. T. & Fedeli, J.-M. Low loss MMI couplers for high performance MZI modulators. IEEE Photonics Technol. Lett. 22, 1485–1487 (2010).
Mikkelsen, J. C., Sacher, W. D. & Poon, J. K. S. Dimensional variation tolerant silicon-on-insulator directional couplers. Opt. Express 22, 3145–3150 (2014).
Gunaydin, L. A. et al. Ultrafast optogenetic control. Nat. Neurosci. 13, 387–392 (2010).
Densmore, A. et al. Compact and low power thermo-optic switch using folded silicon waveguides. Opt. Express 17, 10457–10465 (2009).
Fang, Q. et al. Ultralow power silicon photonics thermo-optic switch with suspended phase arms. IEEE Photonics Technol. Lett. 23, 525–527 (2011).
Maese-Novo, A. et al. Wavelength independent multimode interference coupler. Opt. Express 21, 7033–7040 (2013).
Chen, X., Li, C., Fung, C. K. Y., Lo, S. M. G. & Tsang, H. K. Apodized waveguide grating couplers for efficient coupling to optical fibers. IEEE Photonics Technol. Lett. 22, 1156–1158 (2010).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photon. 7, 205–209 (2013).
Karpov, M., Pfeiffer, M. H. P., Liu, J., Lukashchuk, A. & Kippenberg, T. J. Photonic chip-based soliton frequency combs covering the biological imaging window. Nat. Commun. 9, 1146 (2018).
Halir, R. et al. Ultrabroadband supercontinuum generation in a CMOS-compatible platform. Opt. Lett. 37, 1685–1687 (2012).
Zhao, H. et al. Visible-to-near-infrared octave spanning supercontinuum generation in a silicon nitride waveguide. Opt. Lett. 40, 2177–2180 (2015).
Ikeda, K., Saperstein, R. E., Alic, N. & Fainman, Y. Thermal and Kerr nonlinear properties of plasma-deposited silicon nitride/silicon dioxide waveguides. Opt. Express 16, 12987–12994 (2008).
Ahrens, M. B., Orger, M. B., Robson, D. N., Li, J. M. & Keller, P. J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413–420 (2013).
Pégard, N. C. et al. Compressive light-field microscopy for 3D neural activity recording. Optica 3, 517–524 (2016).
Sanders, J. I. & Kepecs, A. A low-cost programmable pulse generator for physiology and behavior. Front. Neuroeng. 7, 43 (2014).
Kvitsiani, D. et al. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature 498, 363–366 (2013).
Pi, H.-J. et al. Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524 (2013).
Schmitzer-Torbert, N., Jackson, J., Henze, D., Harris, K. & Redish, A. D. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131, 1–11 (2005).
Acknowledgements
This work was supported by the National Science Foundation Brain EAGER (grant no. 1611090) and was performed in part at the Cornell NanoScale Facility, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (grant no. ECCS-1542081). Back-end fabrication processing was done in part at the Advanced Science Research Center NanoFabrication Facility at the Graduate Center of the City University of New York. A.M. was funded by a National Science Foundation Graduate Research Fellowship (grant no. DGE-1144153). X.J. acknowledges the China Scholarship Council for financial support.
Author information
Authors and Affiliations
Contributions
A.M. designed and tested the performance of the nanophotonic probe. Q.L. performed the animal surgery, histological analysis and electrophysiology data analysis. A.M. and Q.L. developed and conducted the in vivo electrophysiology experiment with the assistance of M.A.T. and S.P.R. A.M. fabricated the nanophotonic probe with the assistance of X.J. and J.C. S.P.R. developed and fabricated the integrated recording electrode process. E.S. and G.R.B. assisted with back-end fabrication processing and electrical packaging, respectively. A.M. and M.A.T. developed the fibre packaging for in vivo experiments. S.A.M. developed the software interface for optical characterization. A.M., Q.L., M.A.T., A.K. and M.L. designed the experiment and discussed the results. A.M., Q.L. and M.A.T. designed and built the experimental setup. A.K. and M.L. supervised the project. A.M., Q.L., A.K. and M.L. prepared the manuscript. M.A.T., S.P.R., G.R.B., E.S., X.J., J.C. and S.A.M. edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A.M., Q.L., M.A.T., X.J., A.K. and M.L. are listed as inventors in a patent application related to this work, filed by Columbia University. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussion, figures and references.
Supplementary Video 1
Independent illumination of eight spots with low cross-talk.
Rights and permissions
About this article
Cite this article
Mohanty, A., Li, Q., Tadayon, M.A. et al. Reconfigurable nanophotonic silicon probes for sub-millisecond deep-brain optical stimulation. Nat Biomed Eng 4, 223–231 (2020). https://doi.org/10.1038/s41551-020-0516-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-0516-y
This article is cited by
-
Mlora-CBF: efficient cluster-based routing protocol against resource allocation using modified location routing algorithm with cluster-based flooding
Wireless Networks (2024)
-
The ultra-thin, minimally invasive surface electrode array NeuroWeb for probing neural activity
Nature Communications (2023)
-
Synchronous micromechanically resonant programmable photonic circuits
Nature Communications (2023)
-
A deeper understanding of the brain with photonic–electronic integration
Nature Electronics (2023)
-
Toward implantable devices for angle-sensitive, lens-less, multifluorescent, single-photon lifetime imaging in the brain using Fabry–Perot and absorptive color filters
Light: Science & Applications (2022)