Abstract
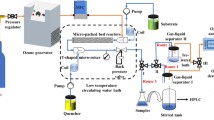
Considering the low conversion and long reaction time in scaling up reaction in batch reactor, the organocatalyzed epoxidation reaction of styrene was intensified under continuous flow conditions using a commercial fluidic reactor (Corning Advanced Flow G1 Reactor). After investigating the effect of reaction temperature, catalyst amount, MeCN/t-BuOH ratio, FO/FAB (buffer solution amount), total feed flow rate and operating mode on the epoxidation reaction, the optimal reaction conditions were identified under continuous flow conditions. Upon optimization, high conversion and excellent selectivity with short reaction time (3.17 min) can be obtained. We successfully developed a process for the rapid and continuous epoxidation of styrene using an organocatalyst with hydrogen peroxide as the oxidant. Compared with the batch conditions, the continuous flow reactor can exhibit unique advantages including high-speed, safety, continuousness and absence of amplifying effect, which will be significant for the industrial production of epoxides.






Similar content being viewed by others
References
McGarrigle EM, Gilheany DG (2005). Chem Rev 105:1563–1602
Davis RL, Stiller J, Naicker T, Jiang H, Jorgensen KA (2014). Angew Chem Int Ed 53:7406–7426
Mizuno N, Yamaguchi K, Kamata K (2005). Coord Chem Rev 249:1944–1956
Tyablikov I, Romanovsky B (2016). Catal Today 278:40–44
Liu JY, Ru MG, Li JX, Jian PM, Wang LX, Jian RQ (2019). Appl Catal B 254:214–222
Han QX, Qi B, Ren WM, He C, Niu JY, Duan CY (2015). Nat Commun 6:10007
White MC, Doyle AG, Jacobsen EN (2001). Am Chem Soc 123:7194–7195
Limnios D, Kokotos CG (2014). J Organomet Chem 79:4270–4276
Lane BS, Burgess K (2003). Chem Rev 103:2457–2473
Triandafillidi I, Tzaras DI, Kokotos CG (2018). ChemCatChem. 10:2521–2535
Shu LH, Shi Y (2000). J Organomet Chem 65:8807–8810
Lifchits O, Reisinger CM, List BJ (2010). J Am Chem Soc 132:10227–10229
Berkessel A, Kramer J, Mummy F, Neudorfl JM, Haag R (2013). Angew Chem Int Ed 452:739–743
Adam W, Saha-Möller CR, Ganespure PA (2001). Chem Rev 101:3499–3548
Neimann K, Neumann R (2001). Chem Commun 487−488
Gemoets HPL, Su Y, Shang M, Hessel V, Luque R, Noel T (2016). Chem Soc Rev 45:83–117
Gutmann B, Cantillo D, Kappe CO (2015). Angew Chem Int Ed 54:6688–6728
Woitalka A, Kuhn S, Jensen KF (2014). Chem Eng Sci 116:1–8
Salmi T, Carucci JH, Roche M, Eranen K, Warna J, Murzin D (2013). Chem Eng Sci 87:306–314
Ying Y, Chen G, Zhao Y, Si L, Yuan Q (2008) Chem. Eng Sci 135:209–215
Britton J, Raston CL (2017). Chem Soc Rev 46:1250–1271
Nieves-Remacha MJ, Kulkarni AA, Jensen KF (2013). Ind Eng Chem Res 52:8996–9010
Calabrese GS, Pissavini S (2011). AICHE J 57:828–834
Wojtowicz-Mlochowska H (2017). Arch Org Chem 2:12–58
Triandafillidi I, Kokotos CG (2017). Org Lett 19:106–109
Voutyritsa E, Triandafillidi I, Kokotos CG (2017). Synthesis. 49:917–924
Theodorou A, Kokotos CG (2017). Green Chem 19:670–674
Theodorou A, Kokotos CG (2017). Adv Synth Catal 359:1577–1581
Triandafillidi I, Raftopoulou M, Savvidou A, Kokotos CG (2017). ChemCatChem. 9:4120–4124
Haas TW (1960). Retrospective Theses and Dissertations:2645
Shang MJ, Noël T, Su YH, Hessel V (2017). AICHE J 63:689–697
van der Waal JC, van Bekkum H (1997). J Mol Catal A 124:137–146
Payne GB, Deming PH, Williams PH (1961). J Organomet Chem 26:659–663
Page PCB, Graham AE, Bethell D, Park BK (1993). Synth Commun 23:1507–1514
Yamaguchi K, Mizugaki T, Ebitani K, Kaneda K (1999). New J Chem 23:799–801
Zhang Y, Born SC, Jensen KF (2014). Org Process Res Dev 18:1476–1481
Nieves-Remacha MJ, Kulkarni AA, Jensen KF (2013). Ind Eng Chem Res 52:8996–9010
Nieves-Remacha MJ, Kulkarni AA, Jensen KF (2012). Ind Eng Chem Res 51:16251–16262
Acknowledgements
Project was supported by the National Natural Science Foundation of China (21476049), the Regional Development Project of Fujian Province (2016H4023), the University-Industry Cooperation Project of Fujian Province (2019H6010), the Industrial Technology Joint Innovation Special Project of Fujian Province (FG-2016005) and the Program for New Century Excellent Talents in University of Fujian Province (HG2017-17).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Research highlights:
1. Continuous-flow conditions were developed and optimized for the epoxidation of styrene.
2. High conversion and excellent selectivity can be obtained under continuous flow conditions.
3. Unique advantages including short reaction time (3.17 min), safety, continuousness and absence of amplifying effect will beneficial for the industrial production of epoxides.
Electronic supplementary material
ESM 1
(DOCX 3399 kb)
Rights and permissions
About this article
Cite this article
Yuan, WQ., Zhou, SQ., Jiang, YY. et al. Organocatalyzed styrene epoxidation accelerated by continuous-flow reactor. J Flow Chem 10, 227–234 (2020). https://doi.org/10.1007/s41981-019-00065-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-019-00065-6