Abstract
Pd(AEMP)Cl2 (AEMP = 2-(2-aminoethyl)-1-methylpyrrolidine) was synthesized and characterized by spectral and thermal measurements.[Pd(AEMP)(H2O)2]2+ reacts with amino acid esters (L) to form mixed ligand [Pd(AEMP)L]2+ complexes. The kinetics of the base hydrolysis of [Pd(AEMP)L]2+ was studied by a pH-stat technique and the corresponding rate constants are reported. The coordinated glycine methyl ester is hydrolyzed efficiently, whereas the coordinated methionine- and histidine- methyl esters undergo hydrolysis with a much lower catalytic activity. The catalytic effect is controlled by the mode of coordination of the ester to the Pd(II) complex. Possible mechanisms for these reactions are considered. Activation parameters were determined experimentally for the hydrolysis of the coordinated glycine methyl ester. DFT calculations (B3LYP/def2svp) were applied to gain further insight into the possible mechanism of the base hydrolysis of the amino acid esters. The calculations are discussed in reference to the reported experimental data.
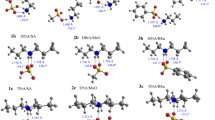
Graphic abstract








Similar content being viewed by others
References
Pesando JM (1975) Proton magnetic resonance studies of carbonic anhydrase. II Group controlling catalytic activity. Biochemistry 14:681–688. https://doi.org/10.1021/bi00675a006
Zenchenko TA, Morozov VN (1995) Mechanical deformation enhances catalytic activity of crystalline carboxypeptidase A. Protein Sci 4:251–257. https://doi.org/10.1002/pro.5560040211
Zhang L, Buchet R, Azzar G (2005) Interactions of caged-ATP and photoreleased ATP with alkaline phosphatase. Biochem Biophys Res Commun 328:591–594. https://doi.org/10.1016/j.bbrc.2005.01.023
Vallee BL, Auld DS (1990) Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 29:5647–5659. https://doi.org/10.1021/bi00476a001
Christianson DW, Lipscomb WN (1989) Carboxypeptidase A. Acc Chem Res 22:62–69. https://doi.org/10.1021/ar00158a003
Harrowfield J, Norris V, Sargeson AM (1976) Reactivity of coordinated nucleophiles. A comparison of metal bound imidazolate and hydroxide ions as models for carbonic anhydrase. J Am Chem Soc 98:7282–7289. https://doi.org/10.1021/ja00439a028
Coleman JE, Chlebowski JF (1979) In: Eichhorn GL, Marzilli LG (eds) Advances in inorganic biochemistry, vol 1. Elsevier, New York, p 1
Jairam R, Lau ML, Adorante J, Potvin PG (2001) Ester hydrolysis with 2,6-di(pyrazol-3-yl)pyridines and their Co(II) complexes in homogeneous and micellar media. J Inorg Biochem 84:113–118. https://doi.org/10.1016/S0162-0134(00)00210-5
Kou X, Meng X, Xie J, Zeng X (2003) Comparative kinetics of carboxylic esters hydrolysis catalyzed by the zinc(II) complex of a macrocyclic Schiff base ligand. Transit Met Chem 28:777–781
Xia J, Li SA, Shi YB, Yu KB, Tang WX (2001) Elucidation of the mechanism of carboxy ester cleavage promoted by a Cu(II) alkoxide complex of a tripodal ligand (N3OX). J Chem Soc Dalton Trans 14:2109–2115
Su X-C, Sun H-W, Zhou Z-F, Lin H-K, Chen L, Zhu S-R, Chen Y-T (2001) Kinetics and mechanism of carboxy ester hydrolysis using Zn(II) complexes with functionalized phenanthroline complexes. Polyhedron 20:91–95. https://doi.org/10.1016/S0277-5387(00)00603-3
Lippard SJ, Berg JM (1994) Principles of bioinorganic chemistry, vol 70. University Science Books, Mill Valley. https://doi.org/10.5860/choice.32-4515
Kurzeev SA, Kazankov GM, Ryabov AD (2000) Increased catalytic activity of primary amine palladacycles in biomimetic hydrolysis of N-t-BOC-S-methionine p-nitrophenyl ester. Inorg Chim Acta 305:1–6. https://doi.org/10.1016/S0020-1693(00)00101-8
Lokhande TN, Bobade AS, Khadse BG (2003) Synthesis of 4-(pyrrolidin anilino)-N-aryl/substituted aryl and heteroaryl succinimides as antimicrobial and antifungal agents. Indian Drugs 40:147–150
Hensler ME, Bernstein G, Nizet V, Nefzi A (2006) Pyrrolidine bis-cyclic guanidines with antimicrobial activity against drug-resistant Gram-positive pathogens identified from a mixture-based combinatorial library. Bioorg Med Chem Lett 16:5073–5079. https://doi.org/10.1016/j.bmcl.2006.07.037
Döndaş HA, Nural Y, Duran N, Kilner C (2006) Synthesis, crystal structure and antifungal/antibacterial activity of some novel highly functionalized benzoyl-aminocarbothioyl pyrrolidines. Turk J Chem 30:573–583
Li X, Li Y, Xu W (2006) Design, synthesis, and evaluation of novel galloyl pyrrolidine derivatives as potential anti-tumor agents. Bioorg Med Chem 14:1287–1293
Imamura S, Ishihara Y, Hattori T, Kurasawa O, Matsushita Y, Sugihara Y, Kanzaki N, Iizawa Y, Baba M, Hashiguchi S (2004) CCR5 antagonists as anti-HIV-1 agents. 1. Synthesis and biological evaluation of 5-oxopyrrolidine-3-carboxamide derivatives. Chem Pharm Bull 52:63–73. https://doi.org/10.1248/cpb.52.63
Obniska J, Zagorska A (2003) Synthesis and anticonvulsant properties of new N-[(4-arylpiperazin-1-yl)-methyl] derivatives of 3-aryl pyrrolidine-2,5-dione and 2-aza-spiro[4.4]nonane-1,3-dione. Farmaco 58:1227–1234. https://doi.org/10.1016/S0014-827X(03)00187-3
Malawska B (2005) New anticonvulsant agents. Curr Top Med Chem 5:69–85. https://doi.org/10.2174/1568026053386944
Colandrea VJ, Legiec IE, Huo P, Yan L, Hale JJ, Mills SG, Bergstrom J, Card D, Chebret G, Hajdu R (2006) 2,5-Disubstituted pyrrolidine carboxylates as potent, orally active sphingosine-1-phosphate (S1P) receptor agonists. Bioorg Med Chem Lett 16:2905–2908. https://doi.org/10.1016/j.bmcl.2006.03.038
Yan L, Budhu R, Huo P, Lynch CL, Hale JJ, Mills SG, Hajdu R, Keohane CA, Rosenbach MJ, Milligan JA (2006) 2-Aryl(pyrrolidin-4-yl)acetic acids are potent agonists of sphingosine-1-phosphate (S1P) receptors. Bioorg Med Chem Lett 16:3564–3568. https://doi.org/10.1016/j.bmcl.2006.03.090
Zhang YJ, Wang Z, Sprous D, Nabioullin R (2006) In silico design and synthesis of piperazine-1-pyrrolidine-2,5-dione scaffold-based novel malic enzyme inhibitors. Bioorg Med Chem Lett 16:525–528. https://doi.org/10.1016/j.bmcl.2005.10.0653
Barrett DG, Catalano JG, Deaton DN, Hassell AM, Long ST, Miller AB, Miller LR, Ray JA, Samano V, Shewchuk LM (2006) Novel, potent P2–P3 pyrrolidine derivatives of ketoamide-based cathepsin K inhibitors. Bioorg Med Chem Lett 16:1735–1739. https://doi.org/10.1016/j.bmcl.2005.11.101
Tran JA, Chen CW, Jiang W, Tucci FC, Fleck BA, Marinkovic D, Arellano M, Chen C (2007) Pyrrolidines as potent functional agonists of the human melanocortin-4 receptor. Bioorg Med Chem Lett 17:5165–5170. https://doi.org/10.1016/j.bmcl.2007.06.088
Shehata MR, Shoukry MM, Nasr FMH, van Eldik R (2008) Complex-formation reactions of dicholoro(S-methyl-l-cysteine)palladium(II) with bio-relevant ligands. Labilization induced by S-donor chelates. Dalton Trans 6:779–786
Shehata MR, Shoukry MM, Abdel-Shakour FH, van Eldik R (2009) Equilibrium studies on complex-formation reactions of Pd[(2-(2-aminoethyl)pyridine)(H2O)2]2+ with ligands of biological significance and displacement reactions of DNA constituents. Eur J Chem 26:3912–3920
Mohamed M, Shoukry AA, Shoukry MM (2006) Int J Chem Kinet 38:737
Mohamed MM, Shoukry MM (2002) Complex formation equilibria of palladium(II) complexes involving N,N′-dimethylethylenediamine, DNA constituents and cyclobutane dicarboxylic acid. The catalysis of glycine methyl ester hydrolysis through complex formation. Polyhedron 21:167–173. https://doi.org/10.1016/S0277-5387(01)00958-5
Shehata MR, Shoukry MM, Shoukry SA, Mabrouk MA (2015) Thermal stability of Pd (1,4-bis (2-hydroxyethyl)piperazine) Cl2 and its role in the catalysis of base hydrolysis of α-amino acid esters. J Coord Chem 68:3272–3281. https://doi.org/10.1080/00958972.2015.1061659
Shoukry MM, Khairy EM, Saeed A (1987) Equilibrium and hydrolysis of α-amino acid esters in mixed-ligand complexes with diethylenetriamine palladium(II). Transit Met Chem 12:315–319. https://doi.org/10.1007/BF01024021
Shehata MR, Shoukry MM, Ragab MS, van Eldik R (2017) Synthesis, characterization, speciation, DNA cleavage, and cytotoxic studies of the Pd[2-(2-aminoethyl)-1-methylpyrrolidine]Cl2 complex with reference to carboplatin. Eur J Inorg Chem 2017:1877–1887. https://doi.org/10.1002/ejic.201601524
Bates RG (1977) Determination of pH: theory and practice, 2nd edn. Wiley Interscience, New York
Hay RW, Banerjee P (1981) Hydrolysis of α-amino-acid esters in mixed-ligand complexes with ethylenediaminepalladium(II). J Chem Soc Dalton Trans 2:362–365. https://doi.org/10.1039/DT9810000362
Hay RW, Basak AK (1982) Hydrolysis of α-amino-acid esters in mixed-ligand complexes with 2,2′-bipyridylpalladium(II). J Chem Soc Dalton Trans 9:1819–1823. https://doi.org/10.1039/DT9820001819
OLIS KIFET (1993) Olis Inc., Borgart, GA, p 19
Mijatović AM, Jelić RM, Bogojeski J, Bugarčić ŽD, Petrović B (2013) Understanding the interactions of diruthenium anticancer agents with amino acids. Monatsh Chem 144:1489–1498
Davis CW (1962) Ion association. Butterworths, London
Robinson RA, Stokes RH (1959) Electrolyte solutions. Butterworths, London
Becke AD (1993) Densityfunctional thermochemistry. III. The role of exact exchange. J Phys Chem 98:5648–5652. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627. https://doi.org/10.1021/j100096a001
Weigend F, Ahlrichs R (2005) Phys Chem Phys Chem 7(18):3297–3305
Schaefer A, Horn H, Ahlrichs R (1992) J Chem Phys 97(4):2571
Schaefer A, Huber C, Ahlrichs R (1994) J Chem Phys 100(8):5829
Eichkorn K, Weigend F, Treutler O, Ahlrichs R (1997) Theor Chem Acc 97:119
Weigend F, Furche F, Ahlrichs R (2003) J Chem Phys 119(24):12753
Barone V, Cossi MJ (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. Phys Chem A 102:1995–2001. https://doi.org/10.1021/jp9716997
Cossi M, Rega N, Scalmani G, Barone VJ (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. Comput Chem 24:669–681. https://doi.org/10.1002/jcc.10189
Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford
Shoukry MM, Khairy EM, El-Sherif AA (2002) Ternary complexes involving copper(II) and amino acids, peptides and DNA constituents. The kinetics of hydrolysis of α-amino acid esters. Transit Met Chem 27:656–664
Bedell SA, Nakon R (1977) Isokinetic temperatures and mechanisms of metal ion promoted hydrolyses of amino acid esters. Inorg Chem 16:3055–3059. https://doi.org/10.1021/ic50178a015
Shoukry MM, Al-Najjar AA, Hosny WM, Abdel Hadi AK, Mahgoub AA, Khalf Alla PA (2010) Kinetics of base hydrolysis of α-amino acid esters catalyzed by [Pd(Et4en)(H2O)2]2+. J Coord Chem 63:2498–2506. https://doi.org/10.1080/00958972.2010.506611
Hay RW, Morris PJ (1973) Interaction of DL-2,3-diaminopropionic acid and its methyl ester with metal ions. Part II. Hydrolysis kinetics. J Chem Soc Dalton Trans 1973:56–61. https://doi.org/10.1039/DT9730000056
Hay RW, Morris PJ (1976) In: Sigel H (ed) Metal ions in biological systems, vol 5. Marcel Dekker, New York, pp 173–243
Hay RW, Banerjee P (1980) Kinetics and mechanism of the base hydrolysis of bis(ethylcysteinato)palladium(II). Inorg Chim Acta 44:1205–1207. https://doi.org/10.1016/S0020-1693(00)91007-7
Acknowledgements
D.C. thanks the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project No. 172011) and the European Union for an Erasmus + Fellowship. The authors gratefully acknowledge the Regionales Rechenzentrum Erlangen (RRZE) for a generous allotment of computer time. We like to thank Prof. Tim Clark for hosting this work at the CCC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shoukry, M.M., Shehata, M.R., Ragab, M.S. et al. Kinetics, mechanism and density functional theory calculations on base hydrolysis of α-amino acid esters catalyzed by [Pd(AEMP)(H2O)2]2+ (AEMP = 2-(2-aminoethyl)-1-methylpyrrolidine). Reac Kinet Mech Cat 129, 613–626 (2020). https://doi.org/10.1007/s11144-020-01734-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01734-7