Abstract
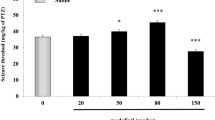
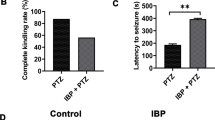
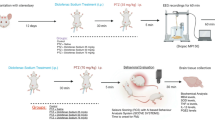
The transient receptor potential vanilloid-1 (TRPV1) receptor has been implicated in the development of epileptic seizures. We examined the effect of the TRPV1 agonist capsaicin on epileptic seizures, neuronal injury and oxidative stress in a model of status epilepticus induced in the rat by intraperitoneal (i.p.) injections of pentylenetetrazole (PTZ). Capsaicin was i.p. given at 1 or 2 mg/kg, 30 min before the first PTZ injection. Other groups were i.p. treated with the vehicle or the anti-epileptic drug phenytoin (30 mg/kg) alone or co-administered with capsaicin at 2 mg/kg. Brain levels of malondialdehyde (MDA), reduced glutathione (GSH), nitric oxide, and paraoxonase-1 (PON-1) activity, seizure scores, latency time and PTZ dose required to reach status epilepticus were determined. Histopathological assessment of neuronal damage was done. Results showed that brain MDA decreased by treatment with capsaicin, phenytoin or capsaicin/phenytoin. Nitric oxide decreased by capsaicin or capsaicin/phenytoin. GSH and PON-1 activity increased after capsaicin, phenytoin or capsaicin/phenytoin. Mean total seizure score decreased by 48.8% and 66.3% by capsaicin compared with 78.7% for phenytoin and 69.8% for capsaicin/phenytoin treatment. Only phenytoin increased the latency (115.7%) and threshold dose of PTZ (78.3%). Capsaicin did not decrease the anti-convulsive effect of phenytoin but prevented the phenytoin-induced increase in latency time and threshold dose. Neuronal damage decreased by phenytoin or capsaicin at 2 mg/kg but almost completely prevented by capsaicin/phenytoin. Thus in this model of status epilepticus, capsaicin decreased brain oxidative stress, the severity of seizures and neuronal injury and its co-administration with phenytoin afforded neuronal protection.









Similar content being viewed by others
Change history
25 February 2020
The original version of this article unfortunately contains an error in the Y axis units in Fig. 1b, c (the symbol µ is not clear: µmol/g.tissue). This has been corrected by publishing this erratum.
References
de Boer HM, Mula M, Sander JW (2008) The global burden and stigma of epilepsy. Epilepsy Behav 12(4):540–546. https://doi.org/10.1016/j.yebeh.2007.12.019
Bowman, Dudek FE, Spitz M (2001) Epilepsy. In: Encyclopedia of life sciences (Wiley Interscience). Nature Publishing Group. http://www.els.net. https://doi.org/10.1038/npg.els.0000100
Stafstrom CE, Carmant L (2015) Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med 5(6):a022426. https://doi.org/10.1101/cshperspect.a022426
Manford M (2017) Recent advances in epilepsy. J Neurol 264:1811–1824. doi:https://doi.org/10.1007/s00415-017-8394-2
Rowley S, Patel M (2013) Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med 62:121–131. doi:https://doi.org/10.1016/j.freeradbiomed.2013.02.002
Geronzi U, Lotti F, Grosso S (2018) Oxidative stress in epilepsy. Expert Rev Neurother 18(5):427–434
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Halliwell B (2009) The wanderings of a free radical. Free Radical Biol Med 46:531–542
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82(2):291–295
Davis RE, Williams M (2012) Mitochondrial function and dysfunction: an update. J Pharmacol Exp Ther 342(3):342:598–607
Chang SJ, Yu BC (2010) Mitochondrial matters of the brain: mitochondrial dysfunction and oxidative status in epilepsy. J Bioenerg Biomembr 42(6):457–459. doi:https://doi.org/10.1007/s10863-010-9317-4
Bhattacharya S (2015) Reactive oxygen species and cellular defense system. In: Rani V, Yadav UCS (eds) Free Radic Hum Health Dis. Springer, New Delhi, pp 17–29. https://doi.org/10.1007/978-81-322-2035-0_2
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Lorigados Pedre L, Gallardo JM, Morales Chacón LM et al (2018) Oxidative stress in patients with drug resistant partial complex seizure. Behav Sci (Basel) 8(6):E59. https://doi.org/10.3390/bs8060059
Lee TH, Lee JG, Yon JM et al (2011) Capsaicin prevents kainic acid-induced epileptogenesis in mice. Neurochem Int 2011 58(6):634–640. doi:https://doi.org/10.1016/j.neuint.2011.01.027
Barrett KT, Wilson RJA, Scantlebury MH (2016) TRPV1 deletion exacerbates hyperthermic seizures in anage-dependent manner in mice. Epilepsy Res 128:27–34
Sun FJ, Guo W, Zheng DH et al (2013) Increased expression of TRPV1 in the cortex and hippocampus from patients with mesial temporal lobe epilepsy. J Mol Neurosci 49(1):182–193. doi:https://doi.org/10.1007/s12031-012-9878-2
Caterina MJ, Schumacher MA, Tominaga M et al (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–824
Nagy I, Friston D, Valente JS et al (2014) Pharmacology of the capsaicin receptor, transient receptor potential vanilloid type-1 ion channel. In: Abdel-Salam OME (ed) Capsaicin as a therapeutic molecule. Progress in drug research, vol 68. Springer, Basel, pp 39–76
Szolcsányi J (2014) Capsaicin and sensory neurones: a historical perspective. In: Abdel-Salam OME (ed) Capsaicin as a therapeutic molecule. Progress in drug research. Springer, Basel, pp 1–37
Steenland HW, Ko SW, Wu LJ et al (2006) Hot receptors in the brain. Mol Pain 2:34
Starowicz K, Cristino L, Di Marzo V (2008) TRPV1 receptors in the central nervous system: potential for previously unforeseen therapeutic applications. Curr Pharm Des 14(1):42–54
Dhir A (2012) Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci.https://doi.org/10.1002/0471142301.ns0937s58
Corda MG, Orlandi M, Giorgi O (1992) Decrease in GABAA receptor function induced by pentylenetetrazol kindling in the rat: role of N-methyl-D-aspartate (NMDA) receptors. Adv Biochem Psychopharmacol 47:235–247
Löscher W, Rogawski MA (2002) Epilepsy. In: Lodge D, Danysz W, Parsons CG (eds) Ionotropic glutamate receptors as therapeutic targets. FP Graham Publishing Co., Johnson, pp 1–42
Pegorini S, Braida D, Verzoni C et al (2005) Capsaicin exhibits neuroprotective effects in a model of transient global cerebral ischemia in Mongolian gerbils. Br J Pharmacol 144:727–735
Chung YC, Baek JY, Kim SR et al (2017) Capsaicin prevents degeneration of dopamine neurons by inhibiting glial activation and oxidative stress in the MPTP model of Parkinson's disease. Exp Mol Med 49(3):e298. doi:https://doi.org/10.1038/emm.2016.159
Erdoğan F, Gölgeli A, Arman F et al (2004) The effects of pentylenetetrazole-induced status epilepticus on behavior, emotional memory, and learning in rats. Epilepsy Behav 5(3):388–393
Sefil F, Kahraman I, Dokuyucu R et al (2014) Ameliorating effect of quercetin on acute pentylenetetrazole induced seizures in rats. Int J Clin Exp Med 7(9):2471–2477
Nair V, Turner GA (1984) The thiobarbituric acid test for lipid peroxidation: structure of the adduct with malondialdehyde. Lipids 19:804–805
Moshage H, Kok B, Huizenga JR et al (1995) Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem 41(6 Pt 1):892–896
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Haagen L, Brock A (1992) A new automated method for phenotyping arylesterase (EC 3.1.1.2) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clin Biochem 30(7):391–395
Drury RVA, Walligton EA (1980) Carltons histological techniques, 5th edn. Oxford University Press, New York
Chen CY, Li W, Qu KP et al (2013) Piperine exerts anti-seizure effects via the TRPV1 receptor in mice. Eur J Pharmacol 714(1–3):288–294. doi:https://doi.org/10.1016/j.ejphar.2013.07.041
Vilela LR, Lima IV, Kunsch ÉB et al (2017) Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav 75:29–35. https://doi.org/10.1016/j.yebeh.2017.07.014
Suemaru K, Yoshikawa M, Aso H et al (2018) TRPV1 mediates the anticonvulsant effects of acetaminophen in mice. Epilepsy Res 145:153–159. doi:https://doi.org/10.1016/j.eplepsyres.2018.06.016
Pezzoli M, Elhamdani A, Camacho S et al (2014) Dampened neural activity and abolition of epileptic-like activity in cortical slices by active ingredients of spices. Sci Rep 4:6825. doi:https://doi.org/10.1038/srep06825
Gutteridge JMC (1995) Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clinc Chem 41:1819–1828
Cnubben NH, Rietjens IM, Wortelboer H et al (2001) The interplay of glutathione-related processes in antioxidant defense. Environ Toxicol Pharmacol 10(4):141–152
Akbas SH, Yegin A, Ozben T (2005) Effect of pentylenetetrazol-induced epileptic seizure on the antioxidant enzyme activities, glutathione and lipid peroxidation levels in rat erythrocytes and liver tissues. Clin Biochem 38(11):1009–1014
Shin HJ, Lee JY, Son EY et al (2007) Curcumin attenuates the kainic acid-induced hippocampal cell death in the mice. Neurosci Lett 416:49–54
Hassanzadeh P, Arbabi E, Rostami F (2014) The ameliorative effects of sesamol against seizures, cognitive impairment and oxidative stress in the experimental model of epilepsy. Iran J Basic Med Sci 17:100–107
Demirbilek S, Ersoy MO, Demirbilek S et al (2004) Small-dose capsaicin reduces systemic inflammatory responses in septic rats. Anesth Analg 99(5):1501–1507
Abdel-Salam OME, Abdel-Rahman RF, Sleem AA et al (2012) Modulation of lipopolysaccharide-induced oxidative stress by capsaicin. Inflammopharmacology 20(4):207–217
Lee CY, Kim M, Yoon SW et al (2003) Short-term control of capsaicin on blood and oxidative stress of rats in vivo. Phytother Res 17(5):454–458
Abdel-Salam OME, Sleem AA, Sayed MAEM (2018) Cannabis sativa increases seizure severity and brain lipid peroxidation in pentylenetetrazole-induced kindling in rats. Biomed Pharmacol J 11(3):1187–1197
Abdel-Salam OME, Sleem AA, Sayed MAEM (2019) Neuroprotective effects of low dose anandamide in pentylenetetrazole-induced kindling in rats. Biomed Pharmacol J 12(1):25–40
Brown GC (2010) Nitric oxide and neuronal death. Nitric Oxide 23(3):153–165
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837. doi:https://doi.org/10.1093/eurheartj/ehr304. 37a–37d.
Wink DA, Mitchell JB (1998) Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 25(4–5):434–456
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87(1):315–424
Han D, Yamada K, Senzaki K et al (2000) Involvement of nitric oxide in pentylenetetrazole-induced kindling in rats. J Neurochem 74(2):792–798
La Du BN (1992) Human serum paraoxonase: arylesterase. Pergamon Press, New York
Précourt LP, Amre D, Denis MC et al (2011) The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis 214:20–36
Mackness M, Mackness B (2013) Targeting paraoxonase-1 in atherosclerosis. Expert Opin Ther Targets 17(7):829–837. doi:https://doi.org/10.1517/14728222.2013.790367
Ferré N, Camps J, Prats E et al (2002) Serum paraoxonase activity: a new additional test for the improved evaluation of chronic liver damage. Clin Chem 48(2):261–268
Menini T, Gugliucci A (2014) Paraoxonase 1 in neurological disorders. Redox Rep 19(2):49–58. doi:https://doi.org/10.1179/1351000213Y.0000000071
Nguyen SD, Sok DE (2004) Preferential inhibition of paraoxonase activity of human paraoxonase 1 by negatively charged lipids. J Lipid Res 45(12):2211–2220. doi:https://doi.org/10.1194/jlr.M400144-JLR200
Emoto MC, Yamato M, Sato-Akaba H et al (2015) Brain redox imaging in the pentylenetetrazole (PTZ)-induced kindling model of epilepsy by using in vivo electron paramagnetic resonance and a nitroxide imaging probe. Neurosci Lett 608:40–44. https://doi.org/10.1016/j.neulet.2015.10.008
Pohle W, Becker A, Grecksch G et al (1997) Piracetam prevents pentylenetetrazol kindling-induced neuronal loss and learning deficits. Seizure 6(6):467–474
Saria A. Skofi tsch G, Lembeck F (1982) Distribution of capsaicin in rat tissues after systemic administration. J Pharm Pharmacol 34:273–275
Frey E, Karney-Grobe S, Krolak T, Milbrandt J, DiAntonio A (2018) TRPV1 agonist, capsaicin, induces axon outgrowth after injury via Ca2+/PKA signaling. Eneuro.https://doi.org/10.1523/ENEURO.0095-18.2018
Gibson HE, Edwards JG, Page RS et al (2008) TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57:746–759
Dobolyi A, Kékesi KA, Juhász G et al (2014) Neuropeptides in epilepsy. Curr Med Chem 21:1–24
Wang XF, Ge TT, Fan J et al (2017) The role of substance P in epilepsy and seizure disorders. Oncotarget 8(44):78225–78233
Acknowledgements
This works is was not supported by research grants
Funding
None. This work was not supported by research grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no potential conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The experiments were conducted in accordance with the ethical guidelines for care, use and handling laboratory animals by the Ethics Committee of the NRC and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to an error in Y axis units in Figure 1B and 1C.
Rights and permissions
About this article
Cite this article
Abdel-Salam, O.M.E., Sleem, A.A., Sayed, M.A.E.B.M. et al. Capsaicin Exerts Anti-convulsant and Neuroprotective Effects in Pentylenetetrazole-Induced Seizures. Neurochem Res 45, 1045–1061 (2020). https://doi.org/10.1007/s11064-020-02979-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-02979-3