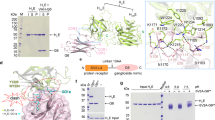
Abstract
Surgical resection is the only cure for neuroendocrine tumors (NETs). However, widespread metastases have already occured by the time of initial diagnosis in many cases making complete surgical removal impossible. We developed a recombinant heavy-chain receptor binding domain (rHCR) of botulinum neurotoxin type A that can specifically target synaptic vesicle 2 (SV2), a surface receptor abundantly expressed in multiple neuroendocrine tumors. Expression of neuroendocrine differentiation markers chromogranin A (CgA) and achaete-scute complex 1 (ASCL1) were signficantly reduced when treated with rHCR. rHCR conjugated to the antimitotic agent monomethyl auristatin E (MMAE) significantly suppressed proliferation of pancreatic carcinoid (BON) and medullary thyroid cancer cells (MZ) at concentrations of 500 and 300 nM respectively, while no growth suppression was observed in pulmonary fibroblasts and cortical neuron control cell lines. In vivo, rHCR-MMAE significantly reduced tumor volume in mouse xenografts with no observed adverse effects. These data suggest recombinant HCR (rHCR) of BoNT/A preferentially targets neuroendocrine cancer without the neurotoxicity of the full BoNT/A and that SV2 is a specific and promising target for delivering drugs to neuroendocrine tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Barakat MT, Meeran K, Bloom SR. Neuroendocrine tumours. Endocr Relat Cancer. 2004;11:1–18.
Chen CQ, Chen HL, Cai SR, Wang Z, Ma JP, Zhang CH, et al. [Clinicopathologic features, diagnosis and treatment of 38 neuroendocrine carcinoma in the digestive system]. Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13:587–9.
Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist. 2008;13:1255–69.
Massironi S, Rossi RE, Casazza G, Conte D, Ciafardini C, Galeazzi M, et al. Chromogranin A in diagnosing and monitoring patients with gastroenteropancreatic neuroendocrine neoplasms: a large series from a single institution. Neuroendocrinology. 2014;100:240–9.
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80(Suppl 1):3–7.
Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–92.
Demirkan BH, Eriksson B. Systemic treatment of neuroendocrine tumors with hepatic metastases. Turk J Gastroenterol. 2012;23:427–37.
Barbieri F, Albertelli M, Grillo F, Mohamed A, Saveanu A, Barlier A, et al. Neuroendocrine tumors: insights into innovative therapeutic options and rational development of targeted therapies. Drug Discov Today. 2014;19:458–68.
Gulenchyn KY, Yao X, Asa SL, Singh S, Law C. Radionuclide therapy in neuroendocrine tumours: a systematic review. Clin Oncol (R Coll Radiol). 2012;24:294–308.
Righi L, Volante M, Tavaglione V, Bille A, Daniele L, Angusti T, et al. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinicopathologic and immunohistochemical study of 218 ‘clinically aggressive’ cases. Ann Oncol. 2010;21:548–55.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP. et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30.
Barbieri JS, Seshasai R, Shemesh A, Sedrak M, Hoffman B, Alley EW. Thymic neuroendocrine tumor presenting with the ectopic ACTH syndrome. J Thorac Oncol. 2013;8:e57–8.
Grillo F, Florio T, Ferrau F, Kara E, Fanciulli G, Faggiano A, et al. Emerging multitarget tyrosine kinase inhibitors in the treatment of neuroendocrine neoplasms. Endocr Relat Cancer. 2018;25:R453–66.
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23.
Pool SE, Bison S, Koelewijn SJ, van der Graaf LM, Melis M, Krenning EP, et al. mTOR inhibitor RAD001 promotes metastasis in a rat model of pancreatic neuroendocrine cancer. Cancer Res. 2013;73:12–8.
Mohammed TA, Holen KD, Jaskula-Sztul R, Mulkerin D, Lubner SJ, Schelman WR, et al. A pilot phase II study of valproic acid for treatment of low-grade neuroendocrine carcinoma. Oncologist. 2011;16:835–43.
Dasari A, Phan A, Gupta S, Rashid A, Yeung SC, Hess K, et al. Phase I study of the anti-IGF1R antibody cixutumumab with everolimus and octreotide in advanced well-differentiated neuroendocrine tumors. Endocr Relat Cancer. 2015;22:431–41.
Portela-Gomes GM, Lukinius A, Grimelius L. Synaptic vesicle protein 2, A new neuroendocrine cell marker. Am J Pathol. 2000;157:1299–309.
Jakobsen AM, Ahlman H, Wangberg B, Kolby L, Bengtsson M, Nilsson O. Expression of synaptic vesicle protein 2 (SV2) in neuroendocrine tumours of the gastrointestinal tract and pancreas. J Pathol. 2002;196:44–50.
Nilsson O, Jakobsen AM, Kolby L, Bernhardt P, Forssell-Aronsson E, Ahlman H. Importance of vesicle proteins in the diagnosis and treatment of neuroendocrine tumors. Ann N Y Acad Sci. 2004;1014:280–3.
Chang WP, Sudhof TC. SV2 renders primed synaptic vesicles competent for Ca2 + -induced exocytosis. J Neurosci. 2009;29:883–97.
Hou JC, Min L, Pessin JE. Insulin granule biogenesis, trafficking and exocytosis. Vitam Horm. 2009;80:473–506.
Keller JE, Cai F, Neale EA. Uptake of botulinum neurotoxin into cultured neurons. Biochemistry. 2004;43:526–32.
Masuyer G, Chaddock JA, Foster KA, Acharya KR. Engineered botulinum neurotoxins as new therapeutics. Annu Rev Pharmacol Toxicol. 2014;54:27–51.
Montal M. Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem. 2010;79:591–617.
Moskowitz CH, Walewski J, Nademanee A, Masszi T, Agura E, Holowiecki J, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132:2639–42.
Almhanna K, Wright D, Mercade TM, Van Laethem JL, Gracian AC, Guillen-Ponce C, et al. A phase II study of antibody-drug conjugate, TAK-264 (MLN0264) in previously treated patients with advanced or metastatic pancreatic adenocarcinoma expressing guanylyl cyclase C. Invest New Drugs. 2017;35:634–41.
Ott PA, Hamid O, Pavlick AC, Kluger H, Kim KB, Boasberg PD, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with advanced melanoma. J Clin Oncol. 2014;32:3659–66.
Arsenault J, Ferrari E, Niranjan D, Cuijpers SA, Gu C, Vallis Y, et al. Stapling of the botulinum type A protease to growth factors and neuropeptides allows selective targeting of neuroendocrine cells. J Neurochem. 2013;126:223–33.
Xu N, Ma C, Ou J, Sun WW, Zhou L, Hu H, et al. Comparative Proteomic Analysis of Three Chinese Hamster Ovary (CHO) Host Cells. Biochem Eng J. 2017;124:122–9.
Xu N, Ou J, Gilani A-K, Zhou L, Liu M. High-level expression of recombinant IgG1 by CHO K1 platform. Frontiers of Chemical Science and Engineering. 2015;9:376–80.
Polakis P. Antibody drug conjugates for cancer therapy. Pharmacol Rev. 2016;68:3–19.
Zarebczan B, Pinchot SN, Kunnimalaiyaan M, Chen H. Hesperetin, a potential therapy for carcinoid cancer. Am J Surg. 2011;201:329–32.
Khan A, Gillis K, Clor J, Tyagarajan K. Simplified evaluation of apoptosis using the Muse cell analyzer. Postepy Biochem. 2012;58:492–6.
Conover WJ. Practical Nonparametric Statistics. 3rd ed. Hoboken, NJ: Wiley; 1999.
Tilkian AG, Conover MB. Understanding heart sounds and murmurs. xii. Philadelphia: Saunders; 1979. p. 122.
Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70.
Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592–6.
Hong WS, Young EW, Tepp WH, Johnson EA, Beebe DJ. A microscale neuron and Schwann cell coculture model for increasing detection sensitivity of botulinum neurotoxin type A. Toxicol Sci. 2013;134:64–72.
Martins RG, Rajendran JG, Capell P, Byrd DR, Mankoff DA. Medullary thyroid cancer: options for systemic therapy of metastatic disease? J Clin Oncol. 2006;24:1653–5.
Chang CL, Munin MC, Skidmore ER, Niyonkuru C, Huber LM, Weber DJ. Effect of baseline spastic hemiparesis on recovery of upper-limb function following botulinum toxin type A injections and postinjection therapy. Arch Phys Med Rehabil. 2009;90:1462–8.
Nowack A, Yao J, Custer KL, Bajjalieh SM. SV2 regulates neurotransmitter release via multiple mechanisms. Am J Physiol Cell Physiol. 2010;299:C960–7.
Hong WS, Pezzi HM, Schuster AR, Berry SM, Sung KE, Beebe DJ. Development of a highly sensitive cell-based assay for detecting botulinum neurotoxin type A through neural culture media optimization. J Biomol Screen. 2016;21:65–73.
Moskowitz CH, Walewski J, Nademanee A, Masszi T, Agura E, Holowiecki J, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132:2639–42.
Bendell J, Saleh M, Rose AA, Siegel PM, Hart L, Sirpal S, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2014;32:3619–25.
Simpson LL, Rapport MM. The binding of botulinum toxin to membrane lipids: sphingolipids, steroids and fatty acids. J Neurochem. 1971;18:1751–9.
Yowler BC, Kensinger RD, Schengrund CL. Botulinum neurotoxin A activity is dependent upon the presence of specific gangliosides in neuroblastoma cells expressing synaptotagmin I. J Biol Chem. 2002;277:32815–9.
Dong M, Liu H, Tepp WH, Johnson EA, Janz R, Chapman ER. Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin E into neurons. Mol Biol Cell. 2008;19:5226–37.
Acknowledgements
We thank Dr. Eric Johnson, Sabine Pellett and William Tepp at the University of Wisconsin-Madison and Dr. Joseph Barbieri at the Medical College of Wisconsin, Milwaukee, WI for supplying BoNT/A and rHCR for this study. We also thank Drs. Margaret X. Liu and Jianfa Ou from University of Alabama at Birmingham for rHCR-MMAE conjugate.
Funding
This research was supported by the National Research Service Award (NRSA) T32 EB011434 and CCTS Partner Network Multidisciplinary Pilot Program award at UAB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Whitt, J., Hong, W.S., Telange, R.R. et al. Non-toxic fragment of botulinum neurotoxin type A and monomethyl auristatin E conjugate for targeted therapy for neuroendocrine tumors. Cancer Gene Ther 27, 898–909 (2020). https://doi.org/10.1038/s41417-020-0167-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-020-0167-x
This article is cited by
-
Botulinum toxin in cancer therapy—current perspectives and limitations
Applied Microbiology and Biotechnology (2022)
-
Treatment personalization in gastrointestinal neuroendocrine tumors
Current Treatment Options in Oncology (2021)