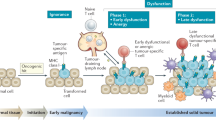
Abstract
The T cell infiltrates that are formed in human cancers are a modifier of natural disease progression and also determine the probability of clinical response to cancer immunotherapies. Recent technological advances that allow the single-cell analysis of phenotypic and transcriptional states have revealed a vast heterogeneity of intratumoural T cell states, both within and between patients, and the observation of this heterogeneity makes it critical to understand the relationship between individual T cell states and therapy response. This Review covers our current knowledge of the T cell states that are present in human tumours and the role that different T cell populations have been hypothesized to play within the tumour microenvironment, with a particular focus on CD8+ T cells. The three key models that are discussed herein are as follows: (1) the dysfunction of T cells in human cancer is associated with a change in T cell functionality rather than inactivity; (2) antigen recognition in the tumour microenvironment is an important driver of T cell dysfunctionality and the presence of dysfunctional T cells can hence be used as a proxy for the presence of a tumour-reactive T cell compartment; (3) a less dysfunctional population of tumour-reactive T cells may be required to drive a durable response to T cell immune checkpoint blockade.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Clemente, C. G. et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77, 1303–1310 (1996).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Zhang, L. et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348, 203–213 (2003).
Fridman, W. H., Pagès, F., Saut’s-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Kvistborg, P. et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci. Transl Med. 6, 254ra128 (2014).
Robert, L. et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin. Cancer Res. 20, 2424–2432 (2014).
Cha, E. et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci. Transl Med. 6, 238ra70 (2014).
Robert, L. et al. Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes. Oncoimmunology 3, e29244 (2014).
Barnes, T. A. & Amir, E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br. J. Cancer 117, 451–460 (2017).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Rosenberg, S. A. et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557 (2011).
Quezada, S. A. et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 207, 637–650 (2010).
Tran, E. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014).
Alspach, E. et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 574, 696–701 (2019).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016). The first single-cell transcriptome and TCR analysis of the TME in human melanoma, dissecting the major T cell states and tumour-specific programmes.
Li, H. et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell 176, 775–789.e18 (2019). Single-cell transcriptome and TCR analysis in human melanoma describing a dysfunctional axis made up of cells that are transcriptionally related and show TCR sharing, as well as a separate cytotoxic population. The dysfunctional T cells identified contain a highly proliferative subset and are indicative of the presence of a tumour-reactive T cell repertoire.
Sade-Feldman, M. et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175, 998–1013.e20 (2018). Single-cell transcriptome analysis in human melanoma indicating that TCF7-expressing T cells in human cancer are associated with response to ICB. Furthermore, the study shows that CD39 − and TIM3 − T cells display reactivity against mouse colon carcinoma cells in the presence of anti-PD1.
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Baitsch, L. et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011).
Thommen, D. S. et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 24, 1–11 (2018). This study defines a T cell subset in human NSCLC with high expression of PD1 that is enriched for tumour reactivity, that expresses markers of T cell activation and that appears predictive of clinical response to anti-PD1 therapy.
Blackburn, S. D., Shin, H., Freeman, G. J. & Wherry, E. J. Selective expansion of a subset of exhausted CD8 T cells by PD-L1 blockade. Proc. Natl Acad. Sci. USA 105, 15016–15021 (2008).
Wherry, E. J., Blattman, J. N., Murali-Krishna, K., van der Most, R. & Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003).
Zheng, C. et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell 169, 1342–1356.e16 (2017). Single-cell transcriptome and TCR analysis of T cells in HCC showing the distribution and connectivity between T cell states in tumour tissue, adjacent normal tissue and blood, and that also provides evidence for the presence of cell state diversity within T cell clones.
Guo, X. et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 24, 978–985 (2018). Single-cell transcriptome analysis of T cells in NSCLC, adjacent normal tissue and blood showing the distribution of T cell states in these tissues and their relatedness based on TCR sharing between cell states inside and outside the tumour. Moreover, this study provides evidence for dysfunctionality as a gradual state.
Yost, K. E. et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25, 1251–1259 (2019). Single cell transcriptome and TCR analysis of T cells in human BCC suggest the presence of novel T cell clonotypes within the dysfunctional (‘exhausted’) compartment after ICB.
Zhang, L. et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 564, 268–272 (2018). Single-cell transcriptome and TCR analysis of T cells in microsatellite-stable and microsatellite-instable colorectal tumours addressing the clonotypic relationships between intratumoural dysfunctional cells and T cells with other cell states that reside in the tumour, normal tissue or blood.
Azizi, E. et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 174, 1293–1308.e36 (2018). Single-cell transcriptome analysis of immune cells in human breast cancer demonstrating that intratumoural T cells reside along a continuum that is driven by activation and terminal differentiation. In addition, cell state diversity is shown both between and within T cell clones.
Clarke, J. et al. Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J. Exp. Med. 216, 2128–2149 (2019). Single-cell transcriptome analysis of T cells in human NSCLC describing a PD1-expressing and TIM3-expressing T RM cell subset that contains a highly proliferative subset and is enriched in lesions of patients responding to anti-PD1 therapy.
Savas, P. et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 24, 986–993 (2018). Single-cell transcriptome analysis of T cells in two human breast cancer samples characterizing T RM cells that express, among other genes, inhibitory receptor genes such as PDCD1 and CTLA4.
Fehlings, M. et al. Checkpoint blockade immunotherapy reshapes the high-dimensional phenotypic heterogeneity of murine intratumoural neoantigen-specific CD8+ T cells. Nat. Commun. 8, 562 (2017).
Chevrier, S. et al. An immune atlas of clear cell renal cell carcinoma. Cell 169, 736–749.e18 (2017).
Giraldo, N. A. et al. Tumor-infiltrating and peripheral blood T-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin. Cancer Res. 23, 4416–4428 (2017).
Wagner, J. et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell 177, 1330–1345.e18 (2019).
Salerno, F. & Wolkers, M. C. T-cells require post-transcriptional regulation for accurate immune responses. Biochem. Soc. Trans. 43, 1201–1207 (2015).
Salerno, F. et al. Critical role of post-transcriptional regulation for IFN-γ in tumor-infiltrating T cells. Oncoimmunology 8, e1532762 (2019).
Dieu-Nosjean, M. C. et al. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol. Rev. 271, 260–275 (2016).
Sautès-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019).
Kunz, D. J., Gomes, T. & James, K. R. Immune cell dynamics unfolded by single-cell technologies. Front. Immunol. 9, 1435 (2018).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019). A mouse melanoma study showing the importance of a low dysfunctional (‘progenitor exhausted’) T cell population marked by TCF1 expression and required for response to anti-PD1 therapy.
Lanitis, E., Dangaj, D., Irving, M. & Coukos, G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann. Oncol. 28, xii18–xii32 (2017).
Scheper, W. et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med. 25, 89–94 (2019). Unbiased analysis of the tumour reactivity of TCRs in human cancer lesions providing formal evidence that human colorectal and ovarian tumours contain high proportions of bystander T cells.
Simoni, Y. et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018). This study demonstrates the presence of virus-specific bystander T cells in human lung and colorectal tumours. Intratumoural bystander T cells were characterized by a lack of CD39 expression, suggesting that CD39 can be used to help distinguish tumour-reactive T cells from bystander cells.
Erkes, D. A. et al. Virus-specific CD8+ T cells infiltrate melanoma lesions and retain function independently of PD-1 expression. J. Immunol. 198, 2979–2988 (2017).
Kvistborg, P. et al. TIL therapy broadens the tumor-reactive CD8+ T cell compartment in melanoma patients. Oncoimmunology 1, 409–418 (2012).
Rosato, P. C. et al. Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat. Commun. 10, 567 (2019).
Pasetto, A. et al. Tumor- and neoantigen-reactive T-cell receptors can be identified based on their frequency in fresh tumor. Cancer Immunol. Res. 4, 734–743 (2016).
Schietinger, A. et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 45, 389–401 (2016).
Ahmadzadeh, M. et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009).
Gros, A. et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 124, 2246–2259 (2014).
Inozume, T. et al. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J. Immunother. 33, 956–964 (2010).
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, (2018). This study identifies CD103 and CD39 as markers of tumour-reactive T cells across multiple human cancer types.
Gubin, M. M. et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515, 577–581 (2014).
Singer, M. et al. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell 166, 1500–1511.e9 (2016).
Gubin, M. M. et al. High-dimensional analysis delineates myeloid and lymphoid compartment remodeling during successful immune-checkpoint cancer therapy. Cell 175, 1014–1030.e19 (2018).
Martínez-Usatorre, A., Donda, A., Zehn, D. & Romero, P. PD-1 blockade unleashes effector potential of both high- and low-affinity tumor-infiltrating T cells. J. Immunol. 201, 792–803 (2018).
Borst, J., Ahrends, T., Bąbała, N., Melief, C. J. M. & Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 18, 635–647 (2018).
Siddiqui, I. et al. Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10 (2019). A mouse study providing evidence for the role of TCF1-expressing PD1 + CD8 + T cells in tumour control on anti-PD1 and anti-CTLA4 combination therapy.
Kurtulus, S. et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1−CD8+ tumor-infiltrating T cells. Immunity 50, 181–194.e6 (2019). A mouse study that describes a PD1-low subset of T cells that responds to ICB and identifies Tcf7 expression to be required for response to anti-PD1 and anti-TIM3 combination therapy.
Utzschneider, D. T. et al. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427 (2016).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Wu, T. et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci. Immunol. 1, eaai8593 (2016).
Spitzer, M. H. et al. Systemic immunity is required for effective cancer immunotherapy. Cell 168, 487–502.e15 (2017).
Brummelman, J. et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8+ T cells infiltrating human tumors. J. Exp. Med. 215, 2520–2535 (2018).
LaFleur, M. W. et al. PTPN2 regulates the generation of exhausted CD8+ T cell subpopulations and restrains tumor immunity. Nat. Immunol. 20, 1335–1347 (2019).
Wei, S. C. et al. Negative co-stimulation constrains T cell differentiation by imposing boundaries on possible cell states. Immunity 50, 1084–1098.e10 (2019).
Daud, A. I. et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J. Clin. Invest. 126, 3447–3452 (2016).
Gide, T. N. et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell 35, 238–255.e6 (2019).
Jiao, S. et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 179, 1177–1190.e13 (2019).
Kim, H. J. & Cantor, H. CD4 T-cell subsets and tumor immunity: the helpful and the not-so-helpful. Cancer Immunol. Res. 2, 91–98 (2014).
Ahrends, T. & Borst, J. The opposing roles of CD4+ T cells in anti-tumour immunity. Immunology 154, 582–592 (2018).
Braumüller, H. et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 494, 361–365 (2013).
Qin, L. et al. Insights into the molecular mechanisms of T follicular helper-mediated immunity and pathology. Front. Immunol. 9, 1884 (2018).
Crotty, S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50, 1132–1148 (2019).
Lavin, Y. et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell 169, 750–765.e17 (2017).
Giordano, M. et al. Molecular profiling of CD 8 T cells in autochthonous melanoma identifies Maf as driver of exhaustion. EMBO J. 34, 2042–2058 (2015).
Li, J., He, Y., Hao, J., Ni, L. & Dong, C. High levels of Eomes promote exhaustion of anti-tumor CD8+ T cells. Front. Immunol. 9, 2981 (2018).
Chihara, N. et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 558, 454–459 (2018).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Paley, M. A. et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225 (2012).
Alfei, F. et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019).
Khan, O. et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 571, 211–218 (2019).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019).
Welch, J. D. et al. Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell 177, 1873–1887.e17 (2019).
Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017).
Wang, C., Singer, M. & Anderson, A. C. Molecular dissection of CD8+ T-cell dysfunction. Trends Immunol. 38, 567–576 (2017).
Philip, M. & Schietinger, A. Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr. Opin. Immunology 58, 98–103 (2019).
Acknowledgements
The authors thank Y. Lubling and M. Logtenberg for input and insightful discussions. This work was supported by ERC AdG SENSIT to T.N.S. and the Dutch Cancer Society Bas Mulder Award to D.S.T.
Author information
Authors and Affiliations
Contributions
A.M.v.d.L researched data for the article. A.M.v.d.L, D.S.T. and T.N.S. jointly discussed data and co-wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks Z. Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Tumour-specific T cell reactivity
-
The capacity of a T cell to recognize tumour cells, regardless of its ability to perform effector function.
- Single-cell RNA sequencing
-
Gene expression profiling method that allows unbiased transcriptome analysis of individual cells.
- Clonotype
-
The unique T cell receptor (TCR) sequence formed by both the TCR α-chain and the TCR β-chain.
- Predictive biomarkers
-
Certain measurements (for example, T cell count or expression level of a marker gene) to make a risk estimate of the response of a patient to therapy.
Rights and permissions
About this article
Cite this article
van der Leun, A.M., Thommen, D.S. & Schumacher, T.N. CD8+ T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer 20, 218–232 (2020). https://doi.org/10.1038/s41568-019-0235-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-019-0235-4
This article is cited by
-
Spatial features of specific CD103+CD8+ tissue-resident memory T cell subsets define the prognosis in patients with non-small cell lung cancer
Journal of Translational Medicine (2024)
-
CDKL1 potentiates the antitumor efficacy of radioimmunotherapy by binding to transcription factor YBX1 and blocking PD-L1 expression in lung cancer
Journal of Experimental & Clinical Cancer Research (2024)
-
cytoviewer: an R/Bioconductor package for interactive visualization and exploration of highly multiplexed imaging data
BMC Bioinformatics (2024)
-
Characterizing and forecasting neoantigens-resulting from MUC mutations in COAD
Journal of Translational Medicine (2024)
-
Single-cell combined bioinformatics analysis: construction of immune cluster and risk prognostic model in kidney renal clear cells based on CD8+ T cell-associated genes
European Journal of Medical Research (2024)