Abstract
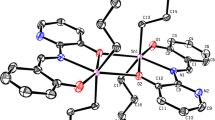
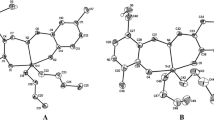
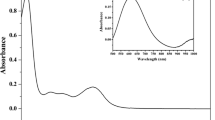
A series of tridentate ONS Schiff bases were synthesised via condensation by reacting 2,3-dihydroxybenzaldehyde with S-2-methylbenzyldithiocarbazate (S2MBDTC) (1), S-4-methylbenzyldithiocarbazate (S4MBDTC) (2) and S-benzyldithiocarbazate (SBDTC) (3) in an equimolar ratio (10 mmol). The Schiff bases were then reacted with diphenyltin(IV) and dimethyltin(IV) dichloride in an equimolar ratio (1 mmol) yielding six new organotin(IV) compounds (4–9). All the compounds were successfully characterised by elemental analysis, FT-IR, multinuclear NMR, UV–Vis, mass spectroscopy and molar conductivity. The molecular geometries for five compounds, 3, 5, 6, 8 and 9, have been established by X-ray crystallography. The five-coordinate geometry for each of the diorganotin molecules was defined by two carbon atoms from the tin-bound substituents as well as three donor atoms derived from the dinegative, tridentate dithiocarbazate ligands, namely thiolate-S, phenoxide-O and imine-N atoms. The resultant five-coordinate C2NOS geometries were intermediate between ideal square pyramidal and trigonal bipyramidal geometries. The diphenyltin(IV) compounds (4–6) exhibited particularly promising and selective cytotoxicity against the A2780 (ovarian), BE2-C (neuroblastoma), SJ-G2 (glioblastoma) and MIA (pancreas) cancer cell lines. The interactions of the compounds (4–9) with calf thymus (CT-DNA) were evaluated using an electronic absorption method, and 7, 8, 9 were found to have good DNA binding affinity. The molecular docking studies of compounds (4–9) with DNA revealed that the compounds interacted with duplex DNA via hydrogen bonding, hydrophobic and electrostatic interactions.
Graphic abstract










Similar content being viewed by others
References
F. Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre, A. Jemal, Cancer J. Clin. 68, 394 (2018)
P. Subbaraj, A. Ramu, N. Raman, J. Dharmaraja, J. Saudi Chem. Soc. 19, 207 (2015)
F. Arjmand, S. Parveen, S. Tabassum, C. Pettinari, Inorg. Chim. Acta 423, 26 (2014)
C. Pellerito, L. Nagy, L. Pellerito, A. Szorcsik, J. Organomet. Chem. 691, 1733 (2006)
R.A. Haque, M.A. Salam, M.A. Arafath, J. Coord. Chem. 68, 2953 (2015)
A.P. Rebolledo, G.M. De Lima, L.N. Gambi, N.L. Speziali, D.F. Maia, C.B. Pinheiro, J.D. Ardisson, M.E. Cortés, H. Beraldo, Appl. Organomet. Chem. 17, 945 (2003)
H.L. Singh, J.B. Singh, S. Bhanuka, Res. Chem. Intermed. 42, 997 (2016)
M.A. Salam, A. Arafath, M.A. Hussein, R. Basri, R. Pervin, Phosphorus Sulfur Silicon Relat. Elem. 191, 1101 (2016)
N. Sonika, R. Malhotra, Phosphorus Sulfur Silicon Relat. Elem. 186, 1449 (2011)
S. Hussain, S. Ali, S. Shahzadi, M.N. Tahir, M. Shahid, J. Coord. Chem. 68, 2369 (2015)
T.S. Basu Baul, A. Paul, L. Pellerito, M. Scopelliti, P. Singh, P. Verma, D. de Vos, Investig. New Drugs 28, 587 (2010)
T. Sedaghat, M. Naseh, H.R. Khavasi, H. Motamedi, Polyhedron 33, 435 (2012)
M. Gielen, Appl. Organomet. Chem. 16, 481 (2002)
A. Gennari, B. Viviani, C.L. Galli, M. Marinovich, R. Pieters, E. Corsini, Toxicol. Appl. Pharmacol. 169, 185 (2000)
S. Tabassum, C. Pettinari, J. Organomet. Chem. 691, 1761 (2006)
R.F. Lee, Mar. Environ. Res. 17, 145 (1985)
F. Wang, H. Yin, J. Cui, Y. Zhang, H. Geng, M. Hong, J. Organomet. Chem. 759, 83 (2014)
T.S. Basu Baul, A. Paul, L. Pellerito, M. Scopelliti, P. Singh, P. Verma, A. Duthie, D. de Vos, E.R.T. Tiekink, Investig. New Drugs 29, 285 (2011)
M. Sirajuddin, S. Ali, M.N. Tahir, Inorg. Chim. Acta 439, 145 (2016)
A. Alama, B. Tasso, F. Novelli, F. Sparatore, Drug Discov. Today 14, 500 (2009)
E.N.M. Yusof, T.B.S.A. Ravoof, J. Jamsari, E.R.T. Tiekink, A. Veerakumarasivam, K.A. Crouse, M.I.M. Tahir, H. Ahmad, Inorg. Chim. Acta 438, 85 (2015)
K.A. Crouse, K.B. Chew, M.T.H. Tarafder, A. Kasbollah, A.M. Ali, B.M. Yamin, H.K. Fun, Polyhedron 23, 161 (2004)
E. Zangrando, M.S. Begum, M.C. Sheikh, R. Miyatake, M.M. Hossain, M.M. Alam, M.A. Hasnat, M.A. Halim, S. Ahmed, M.N. Rahman, A. Ghosh, Arab. J. Chem. 10, 172 (2017)
M.A. Ali, A.H. Mirza, A.L. Tan, L.K. Wei, P.V. Bernhardt, Polyhedron 23, 2405 (2004)
F.N.-F. How, K.A. Crouse, M.I.M. Tahir, M.T.H. Tarafder, A.R. Cowley, Polyhedron 27, 3325 (2008)
T.-J. Khoo, M.K.B. Break, K.A. Crouse, M.I.M. Tahir, A.M. Ali, A.R. Cowley, D.J. Watkin, M.T.H. Tarafder, Inorg. Chim. Acta 413, 68 (2014)
M.H.S.A. Hamid, A.N.A.H. Said, A.H. Mirza, M.R. Karim, M. Arifuzzaman, M. Akbar Ali, P.V. Bernhardt, Inorg. Chim. Acta 453, 742 (2016)
M.A. Ali, M.T.H. Tarafder, J. Inorg. Nucl. Chem. 39, 1785 (1977)
T.B.S.A. Ravoof, K.A. Crouse, M.I.M. Tahir, R. Rosli, D.J. Watkin, F.N.F. How, J. Chem. Crystallogr. 41, 491 (2011)
S.A. Omar, T.B.S.A. Ravoof, M.I.M. Tahir, K.A. Crouse, Transit. Met. Chem. 39, 119 (2013)
E.N.M. Yusof, T.B.S.A. Ravoof, E.R.T. Tiekink, A. Veerakumarasivam, K.A. Crouse, M.I.M. Tahir, H. Ahmad, Int. J. Mol. Sci. 16, 11034 (2015)
Rigaku Oxford Diffraction, CrysAlis PRO (Agilent Technologies Inc., Santa Clara, 2015)
G.M. Sheldrick, Acta Crystallogr. Sect. A Found. Crystallogr. A64, 112 (2008)
G.M. Sheldrick, Acta Crystallogr. Sect. C Struct. Chem. C71, 3 (2015)
L.J. Farrugia, J. Appl. Crystallogr. 45, 849 (2012)
K. Brandenburg, Diamond (Crystal Impact GbR, Bohn, 2006)
A.L. Spek, Acta Crystallogr. Sect. D Biol. Crystallogr. D65, 148 (2009)
E.N.M. Yusof, M.A.M. Latif, M.I.M. Tahir, J.A. Sakoff, M.I. Simone, A.J. Page, A. Veerakumarasivam, E.R.T. Tiekink, T.B.S.A. Ravoof, Int. J. Mol. Sci. 20, 854 (2019)
K. Liu, H. Yan, G. Chang, Z. Li, M. Niu, M. Hong, Inorg. Chim. Acta 464, 137 (2017)
M. Ganeshpandian, R. Loganathan, E. Suresh, A. Riyasdeen, M.A. Akbarsha, M. Palaniandavar, Dalton Trans. 43, 1203 (2014)
R. Loganathan, S. Ramakrishnan, E. Suresh, A. Riyasdeen, M.A. Akbarsha, M. Palaniandavar, Inorg. Chem. 51, 5512 (2012)
E.N.M. Yusof, E.R.T. Tiekink, M.M. Jotani, M.I. Simone, A.J. Page, T.B.S.A. Ravoof, J. Mol. Struct. 1171, 650 (2018)
M.T.H. Tarafder, K. Chew, K.A. Crouse, A.M. Ali, B.M. Yamin, H.K. Fun, Polyhedron 21, 2683 (2002)
P.M. Jeffrey, M. Damian, L. Radom, J. Phys. Chem. A 111, 11683 (2007)
M.A. Ali, S.M.M.-H. Majumder, R.J. Butcher, J.P. Jasinski, J.M. Jasinski, Polyhedron 16, 2749 (1997)
M.A. Ali, A.H. Mirza, R.J. Butcher, Polyhedron 20, 1037 (2001)
R. Malhotra, J.P. Singh, M. Dudeja, K.S. Dhindsa, J. Inorg. Biochem. 46, 119 (1992)
S.A. Elsayed, A.M. Noufal, A.M. El-Hendawy, J. Mol. Struct. 1144, 120 (2017)
Ö. Mıhçıokur, T. Özpozan, J. Mol. Struct. 1149, 27 (2017)
E.N.M. Yusof, M.M. Jotani, E.R.T. Tiekink, T.B.S.A. Ravoof, Acta Crystallogr. Sect. E Crystallogr. Commun. 72, 516 (2016)
M.A. Ali, A.H. Mirza, R.J. Butcher, K.A. Crouse, Transit. Met. Chem. 31, 79 (2006)
Y.F. Naqeebullah, K.M. Chan, L.K. Mun, N.F. Rajab, T.C. Ooi, Molecules 18, 8696 (2013)
J. Holeček, M. Nádvorník, K. Handlíř, A. Lyčka, J. Organomet. Chem. 315, 299 (1986)
M. Sirajuddin, S. Ali, V. Mckee, M. Sohail, H. Pasha, Eur. J. Med. Chem. 84, 343 (2014)
M. Akbar Ali, A.H. Mirza, M.H.S.A. Hamid, P.V. Bernhardt, Polyhedron 24, 383 (2005)
A.W. Addison, T.N. Rao, J. Chem. Soc. Dalton Trans. 7, 1349 (1984)
C.R. Groom, I.J. Bruno, M.P. Lightfoot, S.C. Ward, Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 72, 171 (2016)
Z. Yekke-Ghasemi, R. Takjoo, M. Ramezani, J.T. Mague, RSC Adv. 8, 41795 (2018)
D.P. Malenov, G.V. Janjić, V.B. Medaković, M.B. Hall, S.D. Zarić, Coord. Chem. Rev. 345, 318 (2017)
E.R.T. Tiekink, Coord. Chem. Rev. 345, 209 (2017)
Y. Yang, M. Hong, L. Xu, J. Cui, G. Chang, D. Li, C. Li, J. Organomet. Chem. 804, 48 (2016)
F. Javed, S. Ali, S. Shahzadi, S.K. Sharma, K. Qanungo, K.S. Munawar, I. Khan, Russ. J. Gen. Chem. 87, 2409 (2017)
M. Hong, G. Chang, R. Li, M. Niu, New J. Chem. 40, 7889 (2016)
Acknowledgements
We thank the Department of Chemistry, Faculty of Science and the Molecular Genetics Laboratory and the Department of Biomedical Sciences, Faculty of Medicine and Health Sciences at Universiti Putra Malaysia, Malaysia, as well as the Discipline of Chemistry, University of Newcastle and the Calvary Mater Hospital, Australia, for their facilities. E. N. M. Y wishes to thank Ministry of Higher Education Malaysia for the award of MyPhD, MyBrain15 and the University of Newcastle for the award of University of Newcastle International Postgraduate Research Scholarship. We would also like to thank Karen A. Crouse, Abhi Veerakumarasivam, Michela Simone, Robert Burns and Zalikha Ibrahim for helpful discussions.
Funding
This research was funded by Universiti Putra Malaysia under the Geran Putra IPS (9504600) and Geran Putra IPB (9581001) (UPM) and the Malaysian Fundamental Research Grant Scheme (FRGS No. 01-01-16-1833FR). Crystallographic research at Sunway University was supported by Sunway University Sdn Bhd (Grant. No. STR-RCTR-RCCM-001-2019).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yusof, E.N.M., Ishak, N.N.M., Latif, M.A.M. et al. Selective cytotoxicity of organotin(IV) compounds with 2,3-dihydroxybenzyldithiocarbazate Schiff bases. Res Chem Intermed 46, 2351–2379 (2020). https://doi.org/10.1007/s11164-020-04095-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04095-x