Abstract
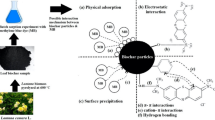
Chitosan (CS) was coalesced with activated charcoal (AC), followed by crosslinking reaction with epichlorohydrin (ECH) to form a mesoporous crosslinked chitosan–epichlorohydrin/activated charcoal composite (CS-ECH/AC). The structural and physicochemical properties of CS-ECH/AC were characterized by Brunauer–Emmett–Teller, X-ray diffraction, scanning electron microscopy, Fourier transform infrared spectroscopy, and point-of-zero charge (pHPZC) analyses. CS-ECH/AC was used to remove thionine (TH), a model cationic dye, from aqueous solution. Batch mode adsorption studies were performed by varying operational adsorption parameters, such as adsorbent dosage (0.04–0.30 g), solution pH (3–11), initial TH dye concentrations (10–100 mg/L), and contact time (0–270 min). The equilibrium data was described well by the Freundlich isotherm, and the maximum adsorption capacity of CS-ECH/AC for TH dye adsorption was 60.9 mg/g at 303 K. The kinetic uptake profiles were well described by the pseudo-second-order model. Thermodynamics results indicated a spontaneous and exothermic adsorption process. The proposed adsorption mechanism included mostly electrostatic attractions, H-bonding interactions, and π–π interactions. All these results showed that CS-ECH/AC can be considered as a feasible biocomposite material for the removal of cationic dyes from wastewater.









Similar content being viewed by others
References
Yan M, Huang W, Li Z (2019) Chitosan cross-linked graphene oxide/lignosulfonate composite aerogel for enhanced adsorption of methylene blue in water. Int J Biol Macromol 136:927–935
Meili L, Lins PVS, Costa MT, Almeida RL, Abud AKS, Soletti JI, Erto A (2019) Adsorption of methylene blue on agroindustrial wastes: experimental investigation and phenomenological modelling. Prog Biophys Mol Biol 141:60–71
Karimifard S, Alavi Moghaddam MR (2016) Removal of an anionic reactive dye from aqueous solution using functionalized multi-walled carbon nanotubes: isotherm and kinetic studies. Desalin. Water Treat. 57(35):16643–16652
Karimifard S, Alavi Moghaddam MR (2016) The effects of microwave regeneration on adsorptive performance of functionalized carbon nanotubes. Water Sci. Technol. 73(11):2638–2643
Wang J, Yao J, Wang L, Xue Q, Pan B (2019) Multivariate optimization of the pulse electrochemical oxidation for treating recalcitrant dye wastewater. Sep. Purif. Technol. 230:115851
Liu J, Liu A, Wang W, Li R, Zhang WX (2019) Feasibility of nanoscale zero-valent iron (nZVI) for enhanced biological treatment of organic dyes. Chemosphere 237:124470
Huang Z, Wang T, Shen M, Huang Z, Chong Y, Cui L (2019) Coagulation treatment of swine wastewater by the method of in-situ forming layered double hydroxides and sludge recycling for preparation of biochar composite catalyst. Chem Eng J 369:784–792
Abdulhameed AS, Mohammad AT, Jawad AH (2019) Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye. J Clean Prod 232:43–56
Li Z, Sellaoui L, Dotto GL, Bonilla-Petriciolet A, Lamine AB (2019) Understanding the adsorption mechanism of phenol and 2-nitrophenol on a biopolymer-based biochar in single and binary systems via advanced modeling analysis. Chem Eng J 371:1–6
Li Z, Dotto GL, Bajahzar A, Sellaoui L, Belmabrouk H, Lamine AB, Bonilla-Petriciolet A (2019) Adsorption of indium (III) from aqueous solution on raw, ultrasound-and supercritical-modified chitin: Experimental and theoretical analysis. Chem Eng J 373:1247–1253
Li Z, Gómez-Avilés A, Sellaoui L, Bedia J, Bonilla-Petriciolet A, Belver C (2019) Adsorption of ibuprofen on organo-sepiolite and on zeolite/sepiolite heterostructure: Synthesis, characterization and statistical physics modeling. Chem Eng J 371:868–875
Jawad AH, Norrahma SSA, Hameed BH, Ismail K (2019) Chitosan-glyoxal film as a superior adsorbent for two structurally different reactive and acid dyes: Adsorption and mechanism study. Int J Biol Macromol 135:569–581
Jawad AH, Nawi MA, Mohamed MH, Wilson LD (2017) Oxidation of chitosan in solution by photocatalysis and product characterization. J Polym Environ 25:828–835
Jawad AH, Mamat NH, Hameed BH, Ismail K (2019) Biofilm of cross-linked Chitosan-Ethylene Glycol Diglycidyl Ether for removal of Reactive Red 120 and Methyl Orange: Adsorption and mechanism studies. J Environ Chem Eng 7:102965
Hydari S, Sharififard H, Nabavinia M, Reza Parvizi M (2012) A comparative investigation on removal performances of commercial activated carbon, chitosan biosorbent and chitosan/activated carbon composite for cadmium. Chem. Eng. J. 193:276–282
Abdulhameed AS, Mohammad AT, Jawad AH (2019) Modeling and mechanism of reactive orange 16 dye adsorption by chitosan-glyoxal/ TiO2 nanocomposite: application of response surface methodology. Desalin Water Treat 164:346–360
Abdulhameed AS, Jawad AH, Mohammad AT (2019) Synthesis of chitosan-ethylene glycol diglycidyl ether/TiO2 nanoparticles for adsorption of reactive orange 16 dye using a response surface methodology approach. Bioresour Technol 293:122071
Zhang L, Sellaoui L, Franco D, Dotto GL, Bajahzar A, Belmabrouk H, Li Z (2020) Adsorption of dyes brilliant blue, sunset yellow and tartrazine from aqueous solution on chitosan: Analytical interpretation via multilayer statistical physics model. Chem Eng J 382:122952
Li Z, Sellaoui L, Dotto GL, Lamine AB, Bonilla-Petriciolet A, Hanafy H, Erto A (2019) Interpretation of the adsorption mechanism of Reactive Black 5 and Ponceau 4R dyes on chitosan/polyamide nanofibers via advanced statistical physics model. J Mol Liq 285:165–170
Nawi MA, Jawad AH, Sabar S, Ngah WW (2011) Photocatalytic-oxidation of solid state chitosan by immobilized bilayer assembly of TiO2–chitosan under a compact household fluorescent lamp irradiation. Carbohydr Polym 83(3):1146–1152
Jawad AH, Islam MA, Hameed BH (2017) Cross-linked chitosan thin film coated onto glass plate as an effective adsorbent for adsorption of reactive orange 16. Int J Biol Macromol 95:743–749
Mohammad AT, Abdulhameed AS, Jawad AH (2019) Box-Behnken design to optimize the synthesis of new crosslinked chitosan-glyoxal/TiO2 nanocomposite: Methyl orange adsorption and mechanism studies. Int J Biol Macromol 129:98–109
Guo M, Wang J, Wang C, Strong PJ, Jiang P, Ok YS, Wang H (2019) Carbon nanotube-grafted chitosan and its adsorption capacity for phenol in aqueous solution. Sci Total Environ 682:340–347
Malek NNA, Jawad AH, Abdulhameed AS, Ismail K, Hameed BH (2020) New magnetic Schiff's base-chitosan-glyoxal/fly ash/Fe3O4 biocomposite for the removal of anionic azo dye: An optimized process. Int J Biol Macromol 146:530–539
Jawad AH, Mubarak NSA, Abdulhameed AS (2020) Tunable Schiff’s base-cross-linked chitosan composite for the removal of reactive red 120 dye: Adsorption and mechanism study. Int J Biol Macromol 142:732–741
Thomas TD (2008) The role of activated charcoal in plant tissue culture. Biotechnol Adv 26(6):618–631
Roy S, Das P, Sengupta S, Manna S (2017) Calcium impregnated activated charcoal: Optimization and efficiency for the treatment of fluoride containing solution in batch and fixed bed reactor. Process Saf Environ 109:18–29
Pinho MT, Silva AM, Fathy NA, Attia AA, Gomes HT, Faria JL (2015) Activated carbon xerogel–chitosan composite materials for catalytic wet peroxide oxidation under intensified process conditions. J Environ Chem Eng 3(2):1243–1251
Sharififard H, Rezvanpanah E, Rad SH (2018) A novel natural chitosan/activated carbon/iron bio-nanocomposite: Sonochemical synthesis, characterization, and application for cadmium removal in batch and continuous adsorption process. Bioresour Technol 270:562–569
Danalıoğlu ST, Bayazit ŞS, Kuyumcu ÖK, Salam MA (2017) Efficient removal of antibiotics by a novel magnetic adsorbent: Magnetic activated carbon/chitosan (MACC) nanocomposite. J Mol Liq 240:589–596
Wróbel-Iwaniec I, Díez N, Gryglewicz G (2015) Chitosan-based highly activated carbons for hydrogen storage. Int J Hydrogen Energy 40(17):5788–5796
Jawad AH, Nawi MA (2012) Characterizations of the photocatalytically-oxidized cross-linked chitosan-glutaraldehyde and its application as a sub-layer in the TiO2/CS-GLA bilayer photocatalyst system. J Polym Environ 20:817–829
Tang C, Hu D, Cao Q, Yan W, Xing B (2017) Silver nanoparticles-loaded activated carbon fibers using chitosan as binding agent: Preparation, mechanism, and their antibacterial activity. Appl Surf Sci 394:457–465
Wang L, Wang Y, Li A, Yang Y, Wang J, Zhao H, Qi T (2014) Electrocatalysis of carbon black-or chitosan-functionalized activated carbon nanotubes-supported Pd with a small amount of La2O3 towards methanol oxidation in alkaline media. Int J Hydrog Energy 39(27):14730–14738
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J Magn Magn Mater 404:179–189
Sing KS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity, (Recommendations 1984), Pure Appl. Chem. 57:603–619
Ahmed MJ, Okoye PU, Hummadi EH, Hameed BH (2019) High-performance porous biochar from the pyrolysis of natural and renewable seaweed (Gelidiella acerosa) and its application for the adsorption of methylene blue. Bioresour Technol 278:159–164
Qi X, Lin L, Shen L, Li Z, Qin T, Qian Y, Shen J (2019) Efficient decontamination of lead ions from wastewater by salecan polysaccharide-based hydrogels. ACS Sustain Chem Eng 7:11014–11023
Qi X, Liu R, Chen M, Li Z, Qin T, Qian Y, Shen J (2019) Removal of copper ions from water using polysaccharide-constructed hydrogels. Carbohydr Polym 209:101–110
Raghunath S, Anand K, Gengan RM, Nayunigari MK, Maity A (2016) Sorption isotherms, kinetic and optimization process of amino acid proline based polymer nanocomposite for the removal of selected textile dyes from industrial wastewater. J Photochem Photobiol B Biol 165:189–201
Njoku VO, Islam MA, Asif M, Hameed BH (2014) Preparation of mesoporous activated carbon from coconut frond for the adsorption of carbofuran insecticide. J Anal Appl Pyrol 110:172–180
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Vet Akad Handl 24:1–39
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Muthukumaran C, Sivakumar VM, Thirumarimurugan M (2016) Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J Taiwan Inst Chem Eng 63:354–362
Qi X, Chen M, Qian Y, Liu M, Li Z, Shen L, Shen J (2019) Construction of macroporous salecan polysaccharide-based adsorbents for wastewater remediation. Int J Biol Macromol 132:429–438
Qi X, Wei W, Su T, Zhang J, Dong W (2018) Fabrication of a new polysaccharide-based adsorbent for water purification. Carbohydr Polym 195:368–377
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Frenudlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Qi X, Li Z, Shen L, Qin T, Qian Y, Zhao S, Shen J (2019) Highly efficient dye decontamination via microbial salecan polysaccharide-based gels. Carbohydr Polym 219:1–11
Agathian K, Kannammal L, Meenarathi B, Kailash S, Anbarasan R (2018) Synthesis, characterization and adsorption behavior of cotton fiber based Schiff base. Int J Biol Macromol 107:1102–1112
Nsabimana A, Kitte SA, Wu F, Qi L, Liu Z, Zafar MN, Xu G (2019) Multifunctional magnetic Fe3O4/nitrogen-doped porous carbon nanocomposites for removal of dyes and sensing applications. Appl Surf Sci 467:89–97
Jawad AH, Mallah SH, Mastuli MS (2018) Adsorption behavior of methylene blue on acid-treated rubber (Hevea brasiliensis) leaf. Desalin Water Treat 124:297–307
Jawad AH, Abdulhameed AS (2020) Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: Adsorption kinetic, isotherm and mechanism study. Surf Interface 18:100422
Dash S, Chaudhuri H, Gupta R, Nair UG (2018) Adsorption study of modified coal fly ash with sulfonic acid as a potential adsorbent for the removal of toxic reactive dyes from aqueous solution: Kinetics and thermodynamics. J Environ Chem Eng 6(5):5897–5905
Rashid RA, Ishak MAM, Hello KM (2018) Adsorptive Removal of Methylene Blue by Commercial Coconut Shell Activated Carbon. Sci Lett 12:27–97
Marrakchi F, Ahmed MJ, Khanday WA, Asif M, Hameed BH (2017) Mesoporous-activated carbon prepared from chitosan flakes via single-step sodium hydroxide activation for the adsorption of methylene blue. Int J Biol Macromol 98:233–239
Madrakian T, Afkhami A, Ahmadi M (2012) Adsorption and kinetic studies of seven different organic dyes onto magnetite nanoparticles loaded tea waste and removal of them from wastewater samples. Spectrochim Acta A Mol Biomol Spectrosc 99:102–109
Madrakian T, Afkhami A, Ahmadi M, Bagheri H (2011) Removal of some cationic dyes from aqueous solutions using magnetic-modified multi-walled carbon nanotubes. J Hazard Mater 196:109–114
Rahmi, Ishmaturrahmi, Mustafa I (2019) Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem J 144:397–402
Jawad AH, Mubarak NSA, Abdulhameed AS (2019) Hybrid crosslinked chitosan epichlorohydrin/TiO2 nanocomposite for reactive red 120 dye adsorption: kinetic, isotherm, thermodynamic, and mechanism study. J Polym Environ 28:624–637
Karaer H, Kaya I (2016) Synthesis, characterization of magnetic chitosan/active charcoal composite and using at the adsorption of methylene blue and reactive blue4. Micropor Mesopor Mater 232:26–38
Acknowledgements
The authors would like to thank the Universiti Teknologi MARA, Institute of Research Management and Innovation (Institut Pengurusan Penyelidikan & Inovasi) for supporting this project under LESTARI grant (600-IRMI 5/3/LESTARI (037/2019)).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jawad, A.H., Abdulhameed, A.S. & Mastuli, M.S. Mesoporous Crosslinked Chitosan-Activated Charcoal Composite for the Removal of Thionine Cationic Dye: Comprehensive Adsorption and Mechanism Study. J Polym Environ 28, 1095–1105 (2020). https://doi.org/10.1007/s10924-020-01671-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01671-5