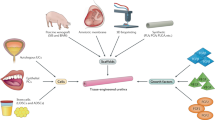
Abstract
Hypospadias is a congenital malformation resulting from the disruption of normal urethral formation with varying global prevalence. Hypospadias repair, especially that of proximal hypospadias (in which reconstruction of a long urethra is necessary), remains a surgical challenge despite more than two decades of surgical technique development and refinement. The lack of tissue substitutes with mechanical and biological properties similar to those of native urethra is a challenge for which the field of tissue engineering might offer promising solutions. However, the use of tissue-engineered constructs in preclinical studies is still hindered by complications such as strictures or fistulae, which have slowed progression to clinical application. Furthermore, the generation of uniform tubular constructs remains a challenge. Exciting advances in the application of nanotechnology and 3D bioprinting to urethral tissue engineering might present solutions to these issues.
Key points
-
Hypospadias is a common congenital abnormality with genetic, molecular and environmental causes that can result in functional or cosmetic issues for affected individuals.
-
Distal hypospadias repair is relatively successful, but complication rates of proximal hypospadias repair are high even for experienced surgeons despite surgical technique refinement.
-
Tissue engineering could address the lack of tissue substitutes with properties similar to those of native urethra for use in urethral reconstruction.
-
For hypospadias in particular, improved understanding of the mechanical properties and biological support being provided by the corpus spongiosum is needed.
-
Amongst the emerging tissue engineering technologies, nanotechnology could enable alteration of the microenvironment to improve wound healing and regeneration, and 3D bioprinting could be used to offer patient-tailored urethral constructs.
-
Scientific barriers, such as identifying the ideal tissue-engineered urethral construct, and practical barriers, including institutional support and funding for translational research, hamper clinical application of tissue-engineered constructs in hypospadias repair.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Li, Y. et al. Canalization of the urethral plate precedes fusion of the urethral folds during male penile urethral development: the double zipper hypothesis. J. Urol. 193, 1353–1359 (2015).
Gong, E. M. & Cheng, E. Y. Current challenges with proximal hypospadias: we have a long way to go. J. Pediatr. Urol. 13, 457–467 (2017).
Baskin, L. et al. Development of the human penis and clitoris. Differentiation 103, 74–85 (2018).
Shafiee, A. & Atala, A. Tissue engineering: toward a new era of medicine. Annu. Rev. Med. 68, 29–40 (2017).
Cunha, G. R., Sinclair, A., Risbridger, G., Hutson, J. & Baskin, L. S. Current understanding of hypospadias: relevance of animal models. Nat. Rev. Urol. 12, 271–280 (2015).
Paulozzi, L. J., Erickson, J. D. & Jackson, R. J. Hypospadias trends in two US surveillance systems. Pediatrics 100, 831–834 (1997).
Springer, A., van den Heijkant, M. & Baumann, S. Worldwide prevalence of hypospadias. J. Pediatr. Urol. 12, 152.e1–152.e7 (2016).
Bergman, J. E. et al. Epidemiology of hypospadias in Europe: a registry-based study. World J. Urol. 33, 2159–2167 (2015).
Schnack, T. H. et al. Familial aggregation of hypospadias: a cohort study. Am. J. Epidemiol. 167, 251–256 (2008).
Carmichael, S. L. et al. Hypospadias and genes related to genital tubercle and early urethral development. J. Urol. 190, 1884–1892 (2013).
Kon, M. et al. Molecular basis of non-syndromic hypospadias: systematic mutation screening and genome-wide copy-number analysis of 62 patients. Hum. Reprod. 30, 499–506 (2015).
Bouty, A., Ayers, K. L., Pask, A., Heloury, Y. & Sinclair, A. H. The genetic and environmental factors underlying hypospadias. Sex. Dev. 9, 239–259 (2015).
Shih, E. M. & Graham, J. M. Jr. Review of genetic and environmental factors leading to hypospadias. Eur. J. Med. Genet. 57, 453–463 (2014).
Dabrowski, E. et al. Proximal hypospadias and a novel WT1 variant: when should genetic testing be considered? Pediatrics 141, S491–S495 (2018).
Giordano, F. et al. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Res. A Clin. Mol. Teratol. 88, 241–250 (2010).
Kalfa, N. et al. Is hypospadias associated with prenatal exposure to endocrine disruptors? A French collaborative controlled study of a cohort of 300 consecutive children without genetic defect. Eur. Urol. 68, 1023–1030 (2015).
Klip, H. et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet 359, 1102–1107 (2002).
Kalfa, N., Paris, F., Soyer-Gobillard, M. O., Daures, J. P. & Sultan, C. Prevalence of hypospadias in grandsons of women exposed to diethylstilbestrol during pregnancy: a multigenerational national cohort study. Fertil. Steril. 95, 2574–2577 (2011).
Rodriguez-Pinilla, E. et al. Risk of hypospadias in newborn infants exposed to valproic acid during the first trimester of pregnancy: a case-control study in Spain. Drug. Saf. 31, 537–543 (2008).
Jentink, J. et al. Valproic acid monotherapy in pregnancy and major congenital malformations. N. Engl. J. Med. 362, 2185–2193 (2010).
Diamanti-Kandarakis, E. et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 30, 293–342 (2009).
Morales-Suarez-Varela, M. M. et al. Parental occupational exposure to endocrine disrupting chemicals and male genital malformations: a study in the Danish National Birth Cohort study. Env. Health 10, 3 (2011).
Estors Sastre, B. et al. Occupational exposure to endocrine-disrupting chemicals and other parental risk factors in hypospadias and cryptorchidism development: a case-control study. J. Pediatr. Urol. 15, 520.e1–520.e8 (2019).
Yinon, Y. et al. Hypospadias in males with intrauterine growth restriction due to placental insufficiency: the placental role in the embryogenesis of male external genitalia. Am. J. Med. Genet. A 152A, 75–83 (2010).
Hussain, N. et al. Hypospadias and early gestation growth restriction in infants. Pediatrics 109, 473–478 (2002).
Glenister, T. W. The origin and fate of the urethral plate in man. J. Anat. 88, 413–425 (1954).
Liu, X. et al. Human glans and preputial development. Differentiation 103, 86–99 (2018).
Duckett, J. W. in Adult and Pediatric Urology 3rd edn (eds Gillenwater, J. Y., Grayhack, J. T., Howards, S. S. & Duckett, J. W.) 2549–2590 (Mosby Year Book, 1996).
Hadidi, A. T. in Hypospadias Surgery (eds Hadidi, A. T. & Azmy, A. F.) 79–82 (Springer, 2004).
Snodgrass, W., Macedo, A., Hoebeke, P. & Mouriquand, P. D. Hypospadias dilemmas: a round table. J. Pediatr. Urol. 7, 145–157 (2011).
van der Horst, H. J. & de Wall, L. L. Hypospadias, all there is to know. Eur. J. Pediatr. 176, 435–441 (2017).
Keays, M. A. et al. Patient reported outcomes in preoperative and postoperative patients with hypospadias. J. Urol. 195, 1215–1220 (2016).
Schlomer, B., Breyer, B., Copp, H., Baskin, L. & DiSandro, M. Do adult men with untreated hypospadias have adverse outcomes? A pilot study using a social media advertised survey. J. Pediatr. Urol. 10, 672–679 (2014).
Jaber, J., Kocherov, S., Chertin, L., Farkas, A. & Chertin, B. Voiding patterns of adult patients who underwent hypospadias repair in childhood. J. Pediatr. Urol. 13, 78.e71–78.e75 (2017).
Chertin, B. et al. Objective and subjective sexual outcomes in adult patients after hypospadias repair performed in childhood. J. Urol. 190, 1556–1560 (2013).
Ortqvist, L. et al. Sexuality and fertility in men with hypospadias; improved outcome. Andrology 5, 286–293 (2017).
Asklund, C. et al. Semen quality, reproductive hormones and fertility of men operated for hypospadias. Int. J. Androl. 33, 80–87 (2010).
Kumar, S. et al. Fertility potential in adult hypospadias. J. Clin. Diagn. Res. 10, PC01–PC05 (2016).
Skarin Nordenvall, A. et al. Psychosocial outcomes in adult men born with hypospadias: a register-based study. PLoS One 12, e0174923 (2017).
Ortqvist, L. et al. Psychosocial outcome in adult men born with hypospadias. J. Pediatr. Urol. 13, 79.e1–79.e7 (2017).
Hadidi, A. T. History of hypospadias: lost in translation. J. Pediatr. Surg. 52, 211–217 (2017).
Baskin, L. S. & Ebbers, M. B. Hypospadias: anatomy, etiology, and technique. J. Pediatr. Surg. 41, 463–472 (2006).
Steven, L. et al. Current practice in paediatric hypospadias surgery; a specialist survey. J. Pediatr. Urol. 9, 1126–1130 (2013).
American Academy of Pediatrics. Timing of elective surgery on the genitalia of male children with particular reference to the risks, benefits, and psychological effects of surgery and anesthesia. Pediatrics 97, 590–594 (1996).
Morrison, C. & Cheng, E. Y. in Operative Techniques in Plastic Surgery (eds Chung, K C., et al.) 3041–3051 (Wolters Kluwer, 2019).
Subramaniam, R., Spinoit, A. F. & Hoebeke, P. Hypospadias repair: an overview of the actual techniques. Semin. Plast. Surg. 25, 206–212 (2011).
Lee, O. T., Durbin-Johnson, B. & Kurzrock, E. A. Predictors of secondary surgery after hypospadias repair: a population based analysis of 5,000 patients. J. Urol. 190, 251–255 (2013).
Wilkinson, D. J., Farrelly, P. & Kenny, S. E. Outcomes in distal hypospadias: a systematic review of the Mathieu and tubularized incised plate repairs. J. Pediatr. Urol. 8, 307–312 (2012).
Pfistermuller, K. L., McArdle, A. J. & Cuckow, P. M. Meta-analysis of complication rates of the tubularized incised plate (TIP) repair. J. Pediatr. Urol. 11, 54–59 (2015).
Hueber, P. A. et al. Long-term functional outcomes of distal hypospadias repair: a single center retrospective comparative study of TIPs, Mathieu and MAGPI. J. Pediatr. Urol. 11, 68 e61–67 (2015).
Liang, W. et al. Surgical repair of mid-shaft hypospadias using a transverse preputial island flap and pedicled dartos flap around urethral orifice. Aesthetic Plast. Surg. 40, 535–539 (2016).
Ahmed, M. & Alsaid, A. Is combined inner preputial inlay graft with tubularized incised plate in hypospadias repair worth doing? J. Pediatr. Urol. 11, 229.e1–229.e4 (2015).
Spinoit, A. F. et al. Grade of hypospadias is the only factor predicting for re-intervention after primary hypospadias repair: a multivariate analysis from a cohort of 474 patients. J. Pediatr. Urol. 11, 70.e1–70.e6 (2015).
Pippi Salle, J. L. et al. Proximal hypospadias: a persistent challenge. Single institution outcome analysis of three surgical techniques over a 10-year period. J. Pediatr. Urol. 12, 28.e1–28.e7 (2016).
Long, C. J. et al. Intermediate-term followup of proximal hypospadias repair reveals high complication rate. J. Urol. 197, 852–858 (2017).
Stanasel, I. et al. Complications following staged hypospadias repair using transposed preputial skin flaps. J. Urol. 194, 512–516 (2015).
Tiryaki, S. et al. Unexpected outcome of a modification of bracka repair for proximal hypospadias: high incidence of diverticula with flaps. J. Pediatr. Urol. 12, 395e1–395.e6 (2016).
Lanciotti, M. et al. Proximal hypospadias repair with bladder mucosal graft: our 10 years experience. J. Pediatr. Urol. 13, 294.e1–294.e6 (2017).
de Kemp, V., de Graaf, P., Fledderus, J. O., Ruud Bosch, J. L. & de Kort, L. M. Tissue engineering for human urethral reconstruction: systematic review of recent literature. PLoS One 10, e0118653 (2015).
Howard, D., Buttery, L. D., Shakesheff, K. M. & Roberts, S. J. Tissue engineering: strategies, stem cells and scaffolds. J. Anat. 213, 66–72 (2008).
Orabi, H. et al. Tissue engineering of urinary bladder and urethra: advances from bench to patients. ScientificWorldJournal 2013, 154564 (2013).
Atala, A. et al. The potential role of tissue-engineered urethral substitution: clinical and preclinical studies. J. Tissue Eng. Regen. Med. 11, 3–19 (2017).
Versteegden, L. R. M. et al. Tissue engineering of the urethra: a systematic review and meta-analysis of preclinical and clinical studies. Eur. Urol. 72, 594–606 (2017).
Versteegden, L. R. et al. Tubular collagen scaffolds with radial elasticity for hollow organ regeneration. Acta Biomater. 52, 1–8 (2017).
Pinnagoda, K. et al. Engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater. 43, 208–217 (2016).
Sack, B. S., Mauney, J. R. & Estrada, C. R. Jr. Silk fibroin scaffolds for urologic tissue engineering. Curr. Urol. Rep. 17, 16 (2016).
Altman, G. H. et al. Silk-based biomaterials. Biomaterials 24, 401–416 (2003).
Chung, Y. G. et al. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS One 9, e91592 (2014).
Lv, X. et al. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials 84, 99–110 (2016).
Jerman, U. D., Veranic, P. & Kreft, M. E. Amniotic membrane scaffolds enable the development of tissue-engineered urothelium with molecular and ultrastructural properties comparable to that of native urothelium. Tissue Eng. Part. C. Methods 20, 317–327 (2014).
Oottamasathien, S., Hotaling, J. M., Craig, J. R., Myers, J. B. & Brant, W. O. Amniotic therapeutic biomaterials in urology: current and future applications. Transl Androl. Urol. 6, 943–950 (2017).
Ramuta, T. Z. & Kreft, M. E. Human amniotic membrane and amniotic membrane-derived cells: how far are we from their use in regenerative and reconstructive urology? Cell Transpl. 27, 77–92 (2018).
Shakeri, S. et al. Application of amniotic membrane as xenograft for urethroplasty in rabbit. Int. Urol. Nephrol. 41, 895–901 (2009).
Gunes, M. et al. A novel approach to penile augmentation urethroplasty using buccal mucosa and amniotic membrane: a pilot study in a rabbit model. Urology 87, 210–215 (2016).
Adamowicz, J. et al. New amniotic membrane based biocomposite for future application in reconstructive urology. PLoS One 11, e0146012 (2016).
El-Assmy, A., El-Hamid, M. A. & Hafez, A. T. Urethral replacement: a comparison between small intestinal submucosa grafts and spontaneous regeneration. BJU Int. 94, 1132–1135 (2004).
Kawano, P. R. et al. Comparative study between porcine small intestinal submucosa and buccal mucosa in a partial urethra substitution in rabbits. J. Endourol. 26, 427–432 (2012).
Huang, J. W. et al. Reconstruction of penile urethra with the 3-dimensional porous bladder acellular matrix in a rabbit model. Urology 84, 1499–1505 (2014).
Cao, N. et al. Prevascularized bladder acellular matrix hydrogel/silk fibroin composite scaffolds promote the regeneration of urethra in a rabbit model. Biomed. Mater. 14, 015002 (2018).
Chun, S. Y. et al. Urethroplasty using autologous urethral tissue-embedded acellular porcine bladder submucosa matrix grafts for the management of long-segment urethral stricture in a rabbit model. J. Korean Med. Sci. 30, 301–307 (2015).
Kajbafzadeh, A. M. et al. The application of tissue-engineered preputial matrix and fibrin sealant for urethral reconstruction in rabbit model. Int. Urol. Nephrol. 46, 1573–1580 (2014).
Kajbafzadeh, A. M. et al. Future prospects for human tissue engineered urethra transplantation: decellularization and recellularization-based urethra regeneration. Ann. Biomed. Eng. 45, 1795–1806 (2017).
Simoes, I. N. et al. Acellular urethra bioscaffold: decellularization of whole urethras for tissue engineering applications. Sci. Rep. 7, 41934 (2017).
Dorin, R. P., Pohl, H. G., De Filippo, R. E., Yoo, J. J. & Atala, A. Tubularized urethral replacement with unseeded matrices: what is the maximum distance for normal tissue regeneration? World J. Urol. 26, 323–326 (2008).
Anwar, H., Dave, B. & Seebode, J. J. Replacement of partially resected canine urethra by polytetrafluoroethylene. Urology 24, 583–586 (1984).
Olsen, L., Bowald, S., Busch, C., Carlsten, J. & Eriksson, I. Urethral reconstruction with a new synthetic absorbable device. An experimental study. Scand. J. Urol. Nephrol. 26, 323–326 (1992).
Lv, X. et al. Electrospun poly(L-lactide)/poly(ethylene glycol) scaffolds seeded with human amniotic mesenchymal stem cells for urethral epithelium repair. Int. J. Mol. Sci. 17, 1262 (2016).
Sartoneva, R. et al. Characterizing and optimizing poly-L-lactide-co-epsilon-caprolactone membranes for urothelial tissue engineering. J. R. Soc. Interface 9, 3444–3454 (2012).
Sartoneva, R. et al. Comparison of a poly-L-lactide-co-epsilon-caprolactone and human amniotic membrane for urothelium tissue engineering applications. J. R. Soc. Interface 8, 671–677 (2011).
Jia, W. et al. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials 69, 45–55 (2015).
Tang, H. et al. Collagen scaffolds tethered with bFGF promote corpus spongiosum regeneration in a beagle model. Biomed. Mater. 13, 031001 (2018).
Nuininga, J. E. et al. Urethral reconstruction of critical defects in rabbits using molecularly defined tubular type I collagen biomatrices: key issues in growth factor addition. Tissue Eng. Part. A 16, 3319–3328 (2010).
Lee, K., Silva, E. A. & Mooney, D. J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface 8, 153–170 (2011).
Fu, Q. & Cao, Y. L. Tissue engineering and stem cell application of urethroplasty: from bench to bedside. Urology 79, 246–253 (2012).
Orabi, H., AbouShwareb, T., Zhang, Y., Yoo, J. J. & Atala, A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: a preclinical study. Eur. Urol. 63, 531–538 (2013).
Zou, Q. & Fu, Q. Tissue engineering for urinary tract reconstruction and repair: progress and prospect in China. Asian J. Urol. 5, 57–68 (2018).
Nagele, U. et al. In vitro investigations of tissue-engineered multilayered urothelium established from bladder washings. Eur. Urol. 54, 1414–1422 (2008).
Sharma, A. K. & Cheng, E. Y. Growth factor and small molecule influence on urological tissue regeneration utilizing cell seeded scaffolds. Adv. Drug. Deliv. Rev. 82–83, 86–92 (2015).
Davis, N. F. et al. Biomaterials and regenerative medicine in urology. Adv. Exp. Med. Biol. 1107, 189–198 (2018).
Panda, A. Stem cell in urology — are we at the cusp of a new era? Transl Androl. Urol. 7, 653–658 (2018).
Peters, E. B. Endothelial progenitor cells for the vascularization of engineered tissues. Tissue Eng. Part. B Rev. 24, 1–24 (2018).
Mikami, H. et al. Two-layer tissue engineered urethra using oral epithelial and muscle derived cells. J. Urol. 187, 1882–1889 (2012).
De Filippo, R. E., Kornitzer, B. S., Yoo, J. J. & Atala, A. Penile urethra replacement with autologous cell-seeded tubularized collagen matrices. J. Tissue Eng. Regen. Med. 9, 257–264 (2015).
Xie, M. et al. Tissue-engineered buccal mucosa using silk fibroin matrices for urethral reconstruction in a canine model. J. Surg. Res. 188, 1–7 (2014).
Wang, F. et al. Urethral reconstruction with tissue-engineered human amniotic scaffold in rabbit urethral injury models. Med. Sci. Monit. 20, 2430–2438 (2014).
Liu, Y. et al. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res. Ther. 8, 63 (2017).
Liu, J. S. et al. Bone marrow stem/progenitor cells attenuate the inflammatory milieu following substitution urethroplasty. Sci. Rep. 6, 35638 (2016).
Wang, Y., Fu, Q., Zhao, R. Y. & Deng, C. L. Muscular tubes of urethra engineered from adipose-derived stem cells and polyglycolic acid mesh in a bioreactor. Biotechnol. Lett. 36, 1909–1916 (2014).
Wang, D. J. et al. Repair of urethral defects with polylactid acid fibrous membrane seeded with adipose-derived stem cells in a rabbit model. Connect. Tissue Res. 56, 434–439 (2015).
Chen, C. et al. Transplantation of amniotic scaffold-seeded mesenchymal stem cells and/or endothelial progenitor cells from bone marrow to efficiently repair 3-cm circumferential urethral defect in model dogs. Tissue Eng. Part. A 24, 47–56 (2018).
Atala, A., Guzman, L. & Retik, A. B. A novel inert collagen matrix for hypospadias repair. J. Urol. 162, 1148–1151 (1999).
Fossum, M., Skikuniene, J., Orrego, A. & Nordenskjold, A. Prepubertal follow-up after hypospadias repair with autologous in vitro cultured urothelial cells. Acta Paediatr. 101, 755–760 (2012).
Orabi, H., Safwat, A. S., Shahat, A. & Hammouda, H. M. The use of small intestinal submucosa graft for hypospadias repair: pilot study. Arab. J. Urol. 11, 415–420 (2013).
Romagnoli, G., De Luca, M., Faranda, F., Franzi, A. T. & Cancedda, R. One-step treatment of proximal hypospadias by the autologous graft of cultured urethral epithelium. J. Urol. 150, 1204–1207 (1993).
Romagnoli, G. et al. Treatment of posterior hypospadias by the autologous graft of cultured urethral epithelium. N. Engl. J. Med. 323, 527–530 (1990).
Raya-Rivera, A. et al. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 377, 1175–1182 (2011).
Ottenhof, S. R. et al. Architecture of the corpus spongiosum: an anatomical study. J. Urol. 196, 919–925 (2016).
Erol, A., Baskin, L. S., Li, Y. W. & Liu, W. H. Anatomical studies of the urethral plate: why preservation of the urethral plate is important in hypospadias repair. BJU Int. 85, 728–734 (2000).
Hayashi, Y. et al. Characterization of the urethral plate and the underlying tissue defined by expression of collagen subtypes and microarchitecture in hypospadias. Int. J. Urol. 18, 317–322 (2011).
Camoglio, F. S., Bruno, C., Zambaldo, S. & Zampieri, N. Hypospadias anatomy: elastosonographic evaluation of the normal and hypospadic penis. J. Pediatr. Urol. 12, 199.e1–199.e5 (2016).
Bhat, A. et al. Comparison of variables affecting the surgical outcomes of tubularized incised plate urethroplasty in adult and pediatric hypospadias. J. Pediatr. Urol. 12, 108.e1–108.e7 (2016).
Feng, C. et al. Evaluation of the biocompatibility and mechanical properties of naturally derived and synthetic scaffolds for urethral reconstruction. J. Biomed. Mater. Res. A 94, 317–325 (2010).
Feng, C., Xu, Y. M., Fu, Q., Zhu, W. D. & Cui, L. Reconstruction of three-dimensional neourethra using lingual keratinocytes and corporal smooth muscle cells seeded acellular corporal spongiosum. Tissue Eng. Part. A 17, 3011–3019 (2011).
Abbas, T. O., Mahdi, E., Hasan, A., AlAnsari, A. & Pennisi, C. P. Current status of tissue engineering in the management of severe hypospadias. Front. Pediatr. 5, 283 (2017).
Mundy, A. R. & Andrich, D. E. Urethral strictures. BJU Int. 107, 6–26 (2011).
Hofer, M. D. et al. Androgen supplementation in rats increases the inflammatory response and prolongs urethral healing. Urology 85, 691–697 (2015).
Ram-Liebig, G. et al. Regulatory challenges for autologous tissue engineered products on their way from bench to bedside in Europe. Adv. Drug. Deliv. Rev. 82–83, 181–191 (2015).
Lu, L. et al. Tissue engineered constructs: perspectives on clinical translation. Ann. Biomed. Eng. 43, 796–804 (2015).
Sharma, P. et al. Aligned fibers direct collective cell migration to engineer closing and nonclosing wound gaps. Mol. Biol. Cell 28, 2579–2588 (2017).
Han, K. et al. EW-7197 eluting nano-fiber covered self-expandable metallic stent to prevent granulation tissue formation in a canine urethral model. PLoS One 13, e0192430 (2018).
Bury, M. I. et al. The promotion of functional urinary bladder regeneration using anti-inflammatory nanofibers. Biomaterials 35, 9311–9321 (2014).
Zhang, K. et al. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 50, 154–164 (2017).
Acknowledgements
The authors thank S. Macomber who contributed to the production of the figures for this article.
Author information
Authors and Affiliations
Contributions
Y.Y.C., M.I.B., E.M.Y., M.D.H. and A.K.S. researched data for the article, Y.Y.C., E.Y.C. and A.K.S. made substantial contributions to discussion of content, Y.Y.C. wrote the manuscript and all authors reviewed and edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks W. Daamen, P. de Graaf and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chan, Y.Y., Bury, M.I., Yura, E.M. et al. The current state of tissue engineering in the management of hypospadias. Nat Rev Urol 17, 162–175 (2020). https://doi.org/10.1038/s41585-020-0281-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-020-0281-4