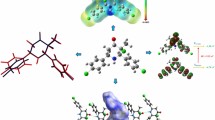
Abstract
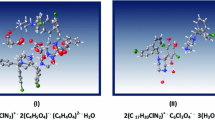
The title compound, C20H22ClNO3, [common name: 3-chloro-3-methyl-r(2),c(6)-bis(p-methoxyphenyl)piperidin-4-one] crystallizes in the P21/c space group with unit cell parameters a = 13.4020(11) Å, b = 7.7888(5) Å and c = 18.1721(14) Å, β = 108.250(9)°, Z = 4. The central piperidin-4-one ring (N1/C1–C5), adopts a slightly distorted chair conformation and an equatorial orientation of all its substituents except for chlorine which is axially located. The dihedral angle between the mean planes of the two phenyl rings is 47.9(4)° and between the piperidin-4- one ring and pendant phenyl rings is 68.8(2)° (C6–C11) and 73.1(6)° (C13–C18), respectively. Crystal packing is stabilized by weak C–H⋯O intermolecular interactions forming chains along the b-axis. Additional weak Cg–π interactions between nearby phenyl rings are also observed. A comparison of these bond lengths and angles within the crystal with Density Functional Theory (DFT) geometry optimized calculations at the B3LYP/6-31+G (d) level has been determined. Hirshfeld surface analysis for determining the molecular shape and visually analyzing the intermolecular interactions in the crystal structure employing 3D molecular surface contours and 2D fingerprint plots gave enrichment ratios for H⋯H, O⋯H, Cl⋯H and C⋯H contacts compared to C–C, Cl⋯Cl and C⋯Cl contacts indicating a higher propensity for O–H interactions to form in this crystal. Electronic transitions have also been predicted by DFT Molecular Orbital calculations and compared to experimental absorption spectra. Molecular orbital diagrams provide visual representations of the top level molecular orbital surfaces in the compound.
Graphical Abstract

Synthesis, crystal structure, DFT geometry optimization and molecular orbital surface calculations and Hirshfeld surface analysis of a new heterocyclic 2,6-disubstituted piperidine-4-one compound.








Similar content being viewed by others
References
Ribeiro da Silva MAV, Cabral JITA (2007) J Therm Anal Calorim 90:865–871
El-Subbagh HI, Abu-Zaid SM, Mahran MA, Badria FA, Al-obaid AM (2000) J Med Chem 43:2915–2921
Mobio IG, Soldatenkov AT, Federov VO, Ageev EA, Sergeeva ND, Lin S, Stashenku EE, Prostakov NS, Andreeva EL (1989) Khim Farm Zh 23:421–427
Katritzky AR, Fan WJ (1990) J Org Chem 55:3205–3209
Parthiban P, Balasubramanian S, Aridoss G, Kabilan S (2009) Bioorg Med Chem Lett 19:2981–2985
Parthiban P, Pallela R, Kim SK, Park DH, Jeong YT (2011) Bioorg Med Chem Lett 21:6678–6686
Casy A, Coates J, Rostron C (1976) J Pharm Pharmacol 28:110
Ramachandran R, Parthiban P, Doddi A, Ramkumar V, Kabilan S (2007) Acta Cryst E63:o4559
Balamurugan S, Thiruvalluvar A, Butcher RJ, Manimekalai A, Jayabharathi J (2008) Acta Cryst E64:o59
Arulraj R, Sivakumar S, Thiruvalluvar A, Manimekalai A (2016) IUCrData, 1, x160188
Arulraj R, Sivakumar S, Kaur M, Thiruvalluvar A, Jasinski JP (2017) Acta Cryst E73:107–111
Thiruvalluvar A, Balamurugan S, Butcher RJ, Manimekalai A, Jayabharathi J (2007) Acta Cryst E63:o4533
Arulraj R, Sivakumar S, Thiruvalluvar A, Kaur M, Jasinski JP (2016) IUCrData, 1, x161580
Arulraj R, Sivakumar S, Thiruvalluvar A, Manimekalai A (2016) IUCrData, 1, x161982
Rigaku Oxford Diffraction (2014) CrysAlis PRO and CrysAlis RED. The Woodlands, TX, USA
Sheldrick GM (2015) Acta Cryst A71:3–8
Sheldrick GM (2015) Acta Cryst C71:3–8
Spek AL (2001) PLATON—a multipurpose crystallographic tool. Ultrecht University, Ultrecht
Johnson CK (1976) ORTEP II. Report ORNL-5138. Oak Ridge National Laboratory, Oak Ridge, TN, USA
Schmidt JR, Polik WF (2007) WebMO Pro, version 8.0.01e; WebMO, LLc: Holland. http://www.webmo.net
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE,. Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RR, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian Inc., Wallingford CT Gaussian 09,Revision D.01
Becke AD (1998) Phys Rev A38:3098
Lee C, Yang W, Parr RG (1988) Phys Rev B37:785
Hehre WJ, Random L, Schleyer PR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Georgakopoulous S, Grondelle RV, Zwan GVD (2004) J Biophys 87:3010–3022
Guzin A (2002) Turk J Chem 26:295–302
Pro IGOR (1988–2009) WaveMetrics, Lake Oswego, Oregon
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Chem Commun 37:3214
McKinnon JJ, Spackman MA, Mitchell AS (2004) Acta Cryst B 60:627
Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, Jayatilaka D, Spackman MA (2017) CrystalExplorer 17.5, University of Western Australia, http://hirshfeldsurface.net
Cremer D, Pople JA (1975) J Am Chem Soc 97:1354–1358
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R (1987) J Chem Soc Perkin Trans 2:S1–S19
Acknowledgements
Authors would like to acknowledge Annamalai University for recording NMR. We extend our thanks to the Principal Dr. P. Kathirvel, Chairman Mr. R. Sattanathan and Treasurer Mr. T. Ramalingam of Thiruvalluvar Arts and Science College for giving permission to carry out research work in the Chemistry Laboratory. JPJ acknowledges the NSF–MRI program (grant No. CHE-1039027) for funds to purchase the X-ray diffractometer.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Arulraj, R., Sivakumar, S., Rajkumar, K. et al. Synthesis, Crystal Structure, DFT Calculations and Hirshfeld Surface Analysis of 3-Chloro-3-methyl-r(2),c(6)-bis(p-methoxyphenyl)piperidin-4-one. J Chem Crystallogr 50, 41–51 (2020). https://doi.org/10.1007/s10870-018-0759-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-018-0759-6