Abstract
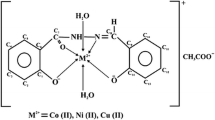
The present study was aimed to synthesize and characterize monometallic complex of Cu(II) using different diamines, i.e., 4-chloro-1,2-phenylenediamine and 4-fluoro-1,2-phenylenediamine, in the presence of CuCl2 to form complexes of the type [Cu(C12H14N4Cl2)]Cl2 and [Cu(C12H14N4FCl)]Cl2. These synthesized complexes were further treated with organotin dichlorides (R2SnCl2), where R = CH3 and C6H5 for the synthesis of heterobimetallic complexes. The complexes have been synthesized using both conventional and microwave heating techniques. The complexes synthesized have been further characterized by elemental analysis, infrared spectra, electronic spectra, ESR, conductance measurement, mass spectra and X-ray powder diffraction studies. On the basis of these studies, distorted octahedral geometry has been proposed for the heterobimetallic complexes synthesized by utilizing monometallic complexes. The newly synthesized complexes have been screened for their antimicrobial activity. The results obtained were compared with the standards used during antifungal and antibacterial activity (i.e., Bavistin and Streptomycin). The pesticidal activity of these complexes was also evaluated against nymph and adult species of Chrotogonus trachypterus by taking into consideration their mortality rate.
Graphic abstract
Environmentally benign synthesis of biologically potent heterobimetallic complexes of copper.






Similar content being viewed by others
References
M.B. Gawande, S.N. Shelke, R. Zboril, R.S. Varma, Acc. Chem. Res. 47, 1338 (2014)
M. Gaba, N. Dhingra, Int. J. Pharm. Educ. Res. 45, 175 (2011)
M. Usharani, E. Akila, P. Jayaseelan, R. Rajavel, IJSER 4, 1055 (2013)
C.A. Puckett, J.K. Barton, Biochemistry 47, 11711 (2008)
J.R. Anacona, K. Mago, J. Camus, Appl. Organomet. Chem. 32, 1 (2018)
Z.C. Liu, B.D. Wang, B. Li, Q. Wang, Z.Y. Yang, T.R. Li, Y. Li, Eur. J. Med. Chem. 45, 5353 (2010)
I. Iakovidis, I. Delimaris, S.M. Piperakis, Mol. Biol. Int. 2011, 1 (2011)
H. Iqbal, S. Ali, S. Shahzadi, S.K. Sharma, K. Qanungo, M. Shahid, J. Coord. Chem. 68, 2434 (2015)
J.O. Adeyemi, D.C. Onwudiwe, Molecules 23, 1 (2018)
R. Vinayak, D. Dey, D. Ghosh, D. Chattopadhyay, A. Ghosh, H.P. Nayek, Appl. Organomet. Chem. 32, 1 (2018)
A. Chaudhary, A.K. Singh, R.V. Singh, J. Inorg. Biochem. 100, 1632 (2006)
F.P. Carvalho, Food Energy Secur. 6, 48 (2017)
A. Singh, A. Chaudhary, Silicon (2018). https://doi.org/10.1007/s12633-018-9971-4
A.I. Vogel, A Textbook of Organic Quantitative Analysis, 5th edn. (Wiley, New York, 2004)
A. Singh, A. Chaudhary, Bioinorg. Chem. Appl. 2018, 1 (2018)
A.I. Vogel, A Textbook of Quantitative Chemical Analysis, 6th edn. (Pearson Education, London, 2006)
A.I. Vogel, A Text Book of Inorganic Analysis (Longmans Green and Co, London, 1968)
N. Fahmi, I. Masih, K. Soni, J. Macromol. Sci. A 52, 548 (2015)
V. Pushpanathan, D.S. Kumar, Int. J. Inorg. Bioinorg. Chem. 3, 35 (2013)
D.J. Finney, Probit Analysis, 3rd edn. (University Press, Cambridge, 1971)
N. Fahmi, S. Shrivastava, R. Meena, S.C. Joshi, R.V. Singh, New J. Chem. 37, 1445 (2013)
S. Yadav, A. Moheman, K.S. Siddiqi, Arab. J. Chem. 9, 1747 (2016)
S. Chandra, M. Tyagi, J. Serb. Chem. Soc. 73, 727 (2008)
J.H. Deshmukh, M.N. Deshpande, ijCEPr 2, 20 (2011)
S. Gunasekaran, S. Seshadri, S. Muthu, Ind. J. Pure Appl. Phys. 44, 581 (2006)
S. Chandra, M. Tyagi, K. Sharma, J. Iran. Chem. Soc. 6, 310 (2009)
K.S. Siddiqi, H. Afaq, S.A.A. Nami, A. Umar, Synth. React. Inorg. Met. Org. Chem. 33, 1459 (2003)
D.P. Singh, K. Kumar, S.S. Dhiman, J. Sharma, J. Enzyme Inhib. Med. Chem. 24, 795 (2009)
R.P.A. Bhoopathy, M. Malathy, R. Jyalakshmi, R. Rajavel, Int. J. Pharm. Biol. Chem. Sci. 5, 11 (2016)
K.S. Siddiqui, F.M.A.M. Aqra, S.A.A. Zaidi, Trans. Met. Chem. 18, 420 (1993)
K. Sharma, N. Fahmi, R.V. Singh, Appl. Organomet. Chem. 15, 221 (2001)
S. Tabassum, M. Zaki, F. Arjmand, I. Ahmad, J. Photochem. Photobiol. B 114, 108 (2012)
S. Chandra, S. Kumar, Spectrochim. Acta A 135, 356 (2015)
A.N.M.A. Alaghaz, Y.A. Ammara, H.A. Bayoumi, S.A. Aldhlmanid, J. Mol. Struct. (2014). https://doi.org/10.1016/j.molstruc.2014.05.078
A.P. Mishra, R.K. Jain, J. Saudi Chem. Soc. 18, 814 (2014)
Acknowledgements
The authors are grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for financial assistance in the form of SRF vide letter No. 09/105(0221)/2015-EMR-I.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Singh, A., Chaudhary, A. Microwave-assisted synthesis, structural elucidation, antimicrobial and pesticidal activity of heterobimetallic complexes of Copper(II). J IRAN CHEM SOC 17, 973–983 (2020). https://doi.org/10.1007/s13738-019-01829-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01829-6