Abstract
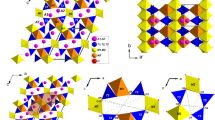
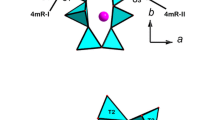
The structural behaviour of maruyamaite (K-dominant tourmaline) X(K0.54Na0.28Ca0.19)Y(Mg1.3Al1.17Fe0.39Ti0.14)Z(Al5Mg)[Si5.95Al0.05O18](BO3)3V,W[O1.69(OH)2.31] from the ultrahigh-pressure metamorphic rocks of Kokchetav massif was studied using synchrotron based single-crystal diffraction up to 20 GPa. Within the whole pressure range the compression is regular and anisotropic, with the c direction being more compressible than the a direction. Fitting the V/P data with the 2nd and 3rd order Birch-Murnaghan equations of state gives: V0 = 1587.2(7) Å3, K0 = 115.6(9) GPa at fixed K′ = 4, and V0 = 1588(1) Å3, K0 = 112(3) GPa, K′ = 4.5(4). The bulk modulus values are slightly higher as compared to those found for dravite and cation-deficient synthetic K-dravite. The pressure evolution of the main structural parameters of K-tourmaline is similar to those of dravite. However, a minor change in the rigidity of local contacts of the X site with 6-membered ring, due to the presence of K, is apparently critical for stabilization of tourmaline structure within 15–20 GPa, which is evinced by the absence of the phase transition observed in dravite near 15.4 GPa. The stabilizing function of K becomes apparent at P > 15 GPa. The comparison of the HP structural behaviour of maruyamaite and dravite supports the recent suggestion that the large X site plays a secondary role in the elastic behaviour of tourmaline, compared to the octahedral framework. In addition, the present study reveals several new features of polyhedra distortions, which demonstrate their complex interaction on compression.







Similar content being viewed by others
References
Agilent (2012) CrysAlis PRO. Agilent Technologies, Yarnton
Angel RJ, Gonzalez-Platas J, Alvaro M (2014) EosFit-7c and a Fortran module (library) for equation of state calculations. Z Kristallogr 229:405–419
Barton R (1969) Refinement of the crystal structure of buergerite and the absolute orientation of tourmalines. Acta Cryst B25:1524–1533
Berryman EJ, Wunder B, Rhede D (2014) Synthesis of K-dominant tourmaline. Am Mineral 99:539–542
Berryman EJ, Wunder B, Wirth R, Rhede D, Schettler G, Franz G, Heinrich W (2015) An experimental study on K and Na incorporation in dravitic tourmaline and insight into the formation environment of diamoniferous tourmaline from the Kokchetav, Massif, Kazakhstan. Contrib Mineral Petrol 169:28
Berryman EJ, Wunder B, Ertl A, Koch-Müller M, Rhede D, Scheidl K, Giester G, Heinrich W (2016) Influence of the X-site composition on tourmaline’s crystal structure: investigation of synthetic K-dravite, dravite, oxy-uvite, and magnesio-foitite using SREF and Raman spectroscopy. Phys Chem Miner 43:83–102
Berryman EJ, Zhang D, Wunder B, Duffy TS (2018) High-pressure compressibility of synthetic tourmaline of near end-member compositions. AGU 2018 Abstracts
Bloodaxe ES, Hughes JM, Dyar MD, Grew ES, Guidotti CV (1999) Linking structure and chemistry in the Schorl-Dravite series. Am Mineral 84:922–928
Capillas C, Tasci ES, de la Flor G, Orobengoa D, Perez-Mato JM, Aroyo MI (2011) A new computer tool at the Bilbao Crystallographic Server to detect and characterize pseudosymmetry. Z Kristallogr 226:186–196
Dietrich RV (1985) The tourmaline group. Van Nostrand Reinhold Company Inc., New York
Dutrow BL, Henry DJ (2011) Tourmaline: A geologic DVD. Elements 7:301–306
Finkelstein GJ, Dera PK, Duffy TS (2015) High-pressure phases of cordierite from single-crystal X-ray diffraction to 15 GPa. Am Mineral 100:1821–1833
Foit FF (1989) Crystal chemistry of alkali-deficient schorl and tourmaline structural relationships. Am Mineral 74:422–431
Gorskaya MG, Frank-Kamenetskaya OV, Rozhdestvenskaya IV, Frank-Kamenetskii VA (1982) Refinement of the crystal structure of Al-rich elbaite, and some aspects of the crystal chemistry of tourmalines. Soviet Physics Crystallogr 27:6
Hawthorne FC (2002) Bond-valence constraints on the chemical composition of tourmaline. Can Mineral 40:789–797
Hawthorne FC, Dirlam DM (2011) Tourmaline, the indicator mineral: From atomic arrangement to Viking navigation. Elements 7:307–312
Hawthorne FC, Henry DJ (1999) Classification of the minerals of the tourmaline group. Eur J Mineral 11:201–215
Hawthorne FC, MacDonald DJ, Burns PC (1993) Reassignment of cation site-occupancies in tourmaline: Al/Mg disorder in the crystal structure of dravite. Am Mineral 78:265–270
Henry DJ, Dutrow BL (1996) Metamorphic tourmaline and its petrologic applications. Rev Mineral 33:503–557
Henry DJ, Novák M, Hawthorne FC, Ertl A, Dutrow BL, Uher P, Pezzotta F (2011) Nomenclature of the tourmaline super-group minerals. Am Mineral 96:895–913
Hezel DC, Kalt A, Marschall HR, Ludwig T, Meyer H-P (2011) Major-element and Li, Be compositional evolution of tourmaline in an Stype granite–pegmatite system and its country rocks: an example from Ikaria, Aegean Sea, Greece. Can Mineral 49:321–340
Hwang SL, Shen P, Chu HT, Yui TF, Liou JG, Sobolev NV, Shatsky VS (2005) Crust-derived potassic fluid in metamorphic microdiamond. Earth Planet Sci Lett 231:295–306
Li H, Qin S, Zhu X, Liu J, Li X, Wu X, Wu Z (2004) In situ high-pressure X-ray diffraction of natural tourmaline. Nuclear techniques 27:919–922
Liebau F (1985) Structural Chemistry of the Silicates. Structure, Bonding, and Classification. Springer-Verlag, Berlin
Likhacheva AY, Rashchenko SV, Seryotkin YV (2012) The deformation mechanism of pressure-induced phase transition in dehydrated analcime. Mineral Mag 76:129–142
Ludwig T, Marschall HR, Pogge von Strandmann PAE, Shabaga BM, Fayek M, Hawthorne FC (2011) A secondary ion mass spectrometry (SIMS) re-evaluation of B and Li isotopic compositions of Cu-bearing elbaite from three global localities. Mineral Mag 75:2485–2494
Lussier AJ, Aguiar PM, Michaelis VK, Kroeker S, Herwig S, Abdu Y, Hawthorne FC (2008) Mushroom elbaite from the Kat Chay mine, Momeik, near Mogok, Myanmar: I. Crystal chemistry by SREF, EMPA, MAS NMR and Mössbauer spectroscopy. Mineral Mag 72:747–761
Lussier AJ, Abdu Y, Hawthorne FC, Michaelis VK, Aguiar PM, Kroeker S (2011) Oscillatory zoned liddicoatite from Anjanabonoina, central Madagascar. I. Crystal chemistry and structure by SREF and 11B and 27Al MAS NMR spectroscopy. Can Mineral 49:63–88
Lussier AJ, Hawthorne FC (2011) Oscillatory zoned liddicoatite from central Madagascar. II. Compositional variations and substitution mechanisms. Can Mineral 49:89–104
Lussier AJ, Ball NA, Hawthorne FC, Henry DJ, Shimizu R, Ogasawara Y, Ota T (2016) Maruyamaite, K(MgAl2)(Al5Mg)Si6O18(BO3)3(OH)3O, a potassium-dominant tourmaline from the ultrahigh-pressure Kokchetav massif, northern Kazakhstan: Description and crystal structure. Am Mineral 101:355–361
MacDonald DJ, Hawthorne FC (1995) The crystal chemistry of Si <−-> Al substitution in tourmaline. Can Mineral 33:849–858
Mao HK, Xu J, Bell PM (1986) Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J Geophys Res 91:4673–4676
Marschall HR, Ludwig T, Altherr R, Kalt A, Tonarini S (2006) Syros metasomatic tourmaline: Evidence for very high-d11B fluids in subduction zones. J Petrol 47:1915–1942
Marschall HR, Jiang S-Y (2011) Tourmaline Isotopes: No element left behind. Elements 7:313–319
Martin RF (2011) Can Mineral 49, pp 1–405
Merlini M, Hafland M (2013) Single-crystal diffraction at megabar conditions by synchrotron radiation. High Pressure Res 33:511–522
Meyer C, Wunder B, Meixner A, Romer RL, Heinrich W (2008) Boron isotope fractionation between tourmaline and fluid: an experimental re-investigation. Contrib Mineral Petrol 156:259–267
Miletich R, Gatta GD, Willi T, Mirwald PW, Lotti P, Merlini M (2014a) Cordierite under hydrostatic compression: anomalous elastic behavior as a precursor for a pressure-induced phase transition. Am Mineral 99:479–493
Miletich R, Scheidl KS, Schmitt M, Moissl AP, Pippinger T, Gatta GD, Schuster B, Trautmann C (2014b) Static elasticity of cordierite I: effect of heavy ion irradiation on the compressibility of hydrous cordierite. Phys Chem Miner 41:579–591
Novák M, Škoda P, Filip J, Macek I, Vaculovič T (2011) Compositional trends in tourmaline from intragranitic NYF pegmatites of the Třebíč Pluton, Czech Republic; electron microprobe, Mössbauer and LA-ICP-MS study. Can Mineral 49:359–380
O’Bannon E, Beavers CM, Kunz M, Williams Q (2018) High-pressure study of dravite tourmaline: Insights into the accommodating nature of the tourmaline structure. Am Mineral 101:1622–1633
O’Bannon E, Williams Q (2016) Beryl-II, a high-pressure phase of beryl: Raman and luminescence spectroscopy to 16.4 GPa. Phys Chem Miner 43:671–687
Ota T, Kobayashi K, Kunihiro T, Nakamura E (2008a) Boron cycling by subducted lithosphere; insights from diamondiferous tourmaline from the Kokchetav ultrahigh-pressure metamorphic belt. Geochim Cosmochim Acta 72:3531–3541
Ota T, Kobayashi K, Katsura T, Nakamura E (2008b) Tourmaline breakdown in a pelitic system: implications for boron cycling through subduction zones. Contrib Mineral Petrol 155:19–32
Pertlik F, Ertl A, Körner W, Brandstätter F, Schuster R (2003) Na-rich dravite in the marbles from Friesach. Chemistry and crystal structure. Neues Jahrbuch für Mineralogie Monatshefte, Carinthia, pp 277–288
Petříček V, Dušek M, Palatinus L (2014) Crystallographic Computing System JANA2006: General features. Zeitschrift für Kristallographie - Crystalline Materials 229:345–352
Prencipe M, Scanavino I, Nestola F, Merlini M, Civalleri B, Bruno M, Dovesi R (2011) High-pressure thermo-elastic properties of beryl (Al4Be6Si12O36) from ab initio calculations, and observations about the source of thermal expansion. Phys Chem Miner 38:223–239
Rothkirch A, Gatta GD, Meyer M, Merkel S, Merlini M, Liermann H-P (2013) Single-crystal diffraction at the Extreme Conditions beamline P02.2: procedure for collecting and analyzing high-pressure single-crystal data. J Synchrotron Radiat 20:711–720
Seryotkin YV, Bakakin VV, Bazhan IS (2005) The structure of dehydrated (Li0.7Na0.3)-analcime: a trigonal deformation of the framework and new low-coordinated non-framework positions. J Struct Chem 46:681–693
Seryotkin YV, Bakakin VV (2008) The thermal behavior of secondary analcime and leucite derivate and its structural interpretation. Russ Geol Geophys 49:207–213
Seryotkin YV, Sokol EV, Bakakin VV, Likhacheva AY (2008) Pyrometamorphic osumilite: occurrence, paragenesis, and crystal structure as compared to cordierite. Eur J Mineral 20:191–198
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32:751–776
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A64:112–122
Schertl H-P, Sobolev NV (2013) The Kokchetav Massif, Kazakhstan: “Type locality” of diamond bearing UHP metamorphic rocks. J Asian Earth Sci 63:5–38
Shimizu R, Ogasawara Y (2005) Discovery of K-tourmaline in diamond- bearing quartz-rich rock from the Kokchetav Massif, Kazakhstan. Mitteilungen der Österreichischen Mineralogischen Gesellschaft 150:141
Shimizu R, Ogasawara Y (2013) Diversity of potassium-bearing tourmalines in diamondiferous Kokchetav UHP metamorphic rocks: a geochemical recorder from peak to retrograde metamorphic stages. J Asian Earth Sci 63:39–55
van Hinsberg V, Henry DJ, Marschall HR (2011) Tourmaline: an ideal indicator of its host environment. Can Mineral 49:1–16
van Hinsberg VJ, Schumacher JC (2007) Using estimated thermodynamic properties to model accessory phases: the case of tourmaline. J Metamorph Geol 25:769–779
Xu J, Kuang Y, Zhang B, Liu Y, Fan D, Li X, Xie H (2016) Thermal equation of state of natural tourmaline at high pressure and temperature. Phys Chem Miner 43:315–326
Acknowledgements
The authors are grateful to J. Cempírek and an anonymous reviewer for their helpful remarks, as well as to Yu.V. Seryotkin for valuable discussion of the results. This study is supported by the Russian Scientific Foundation (project 18-17-00186). Diffraction experiments were carried at the European Synchrotron Radiation Facility and supported by approval of ESRF Proposal ES-810.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: S. W. Faryad
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
F-f plots based on the Birch-Murnaghan 2nd order (a) and 3rd order (b) EoS fit of the pressure volume data for maruyamaite. (PNG 135 kb)
Fig. S2
Pressure dependence of the T6O18 ring ditrigonality in maruyamaite (solid symbols) and dravite (empty symbols, data compiled from O’Bannon et al. 2018) structure. (PNG 32 kb)
Fig. S3
Pressure dependence of the T6O18 ring puckering in maruyamaite (solid symbols) and dravite (empty symbols, data compiled from O’Bannon et al. 2018) structure. (PNG 41 kb)
ESM 1
(ZIP 380 kb)
Rights and permissions
About this article
Cite this article
Likhacheva, A.Y., Rashchenko, S.V., Musiyachenko, K.A. et al. Compressibility and structure behaviour of maruyamaite (K-tourmaline) from the Kokchetav massif at high pressure up to 20 GPa. Miner Petrol 113, 613–623 (2019). https://doi.org/10.1007/s00710-019-00672-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-019-00672-0