Abstract
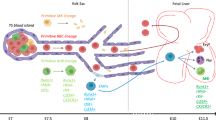
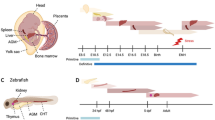
Bone marrow is the main hematopoietic organ of mature mammals. Hematopoietic stem cells (HSCs) are maintained in it throughout an organism’s whole life; it also regulates the production of mature blood cells. Adult HSCs are characterized by polypotency, self-renewal, and a specific phenotype, as well as by the ability to direct migration to the hematopoietic organs. The ability of the HSCs to restore hematopoiesis after transplantation into another organism is the main functional criterion for their existence in tissue. However, HSCs, which function in postnatal ontogenesis and possess these properties, represent the final stage of maturation of their precursors (pre-HSCs), which arise in prenatal development. In embryogenesis, hematopoiesis occurs in several transitional blood-forming organs: the yolk sac, aorta-gonad-mesonephros (AGM), placenta, and liver. Despite the long history of research on hematopoietic system ontogenesis, the anatomical site of the origination of the first pre-HSCs, which give rise to the definitive HSC line, is still not entirely clear. The review summarizes modern concepts of the features of the hematopoietic cells formed in the yolk sac, AGM, and placenta, and their contribution to embryonic liver colonization and in definitive hematopoiesis. Further study of the mechanisms of HSC formation in embryogenesis is of undoubted importance, not only for an understanding of the fundamental aspects of hematopoietic system functioning but also for improvement in treatment methods for hematological diseases.
Similar content being viewed by others
REFERENCES
Al-Drees, M.A., Yeo, J.H., Boumelhem, B.B., et al., Making blood: the haematopoietic niche throughout ontogeny, Stem Cells Int., 2015, vol. 2015, art. ID 571893.https://doi.org/10.1155/2015/571893
Alvarez-Silva, M., Belo-Diabangouaya, P., Salaün, J., and Dieterlen-Lièvre, F., Mouse placenta is a major hematopoietic organ, Development, 2003, vol. 130, no. 22, pp. 5437–5444.
Anckaert, M.A. and Symann, M., In vivo induction of granulopoiesis in visceral yolk-sac cells by fetal hepatic factors, J. Embryol. Exp. Morphol., 1983, vol. 73, pp. 87–95.
Auerbach, R., Huang, H., and Lu, L., Hematopoietic stem cells in the mouse embryonic yolk sac, Stem Cells, 1996, vol. 14, no. 3, pp. 269–280.
Azevedo, P.N., Tavares, G.P., Croy, B.A., and Pelajo-Machado, M., Localization of transient immature hematopoietic cells to two distinct, potential niches in the developing mouse placenta, Placenta, 2016, vol. 47, pp. 1–11.
Bárcena, A., Muench, M.O., Kapidzic, M., and Fisher, S.J., A new role for the human placenta as a hematopoietic site throughout gestation, Reprod. Sci., 2009, vol. 16, no. 2, pp. 178–187.
Baron, M.H., Concise review: early embryonic erythropoiesis: not so primitive after all, Stem Cells, 2013, vol. 31, no. 5, pp. 849–856.
Bhatia, M., Wang, J.C.Y., Kapp, U., et al., Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice, Proc. Natl. Acad. Sci. U.S.A., 1997, vol. 94, no. 10, pp. 5320–5325.
Blair, A. and Thomas, D.B., Preferential adhesion of fetal liver derived primitive haemopoietic progenitor cells to bone marrow stroma, Br. J. Hematol., 1997, vol. 99, no. 4, pp. 726–31.
Chagraoui, J., Lepage-Noll, A., Anjo, A., et al., Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition, Blood, 2003, vol. 101, no. 8, pp. 2973–2982.
Challier, J.C., Galtier, M., Cortez, A., et al., Immunocytological evidence for hematopoiesis in the early human placenta, Placenta, 2005, vol. 26, no. 4, pp. 282–288.
Charbord, P., Oostendorp, R., Pang, W., et al., Comparative study of stromal cell lines derived from embryonic, fetal and postnatal mouse blood-forming tissues, Exp. Hematol., 2002, vol. 30, no. 10, pp. 1202–1210.
Chen, M.J., Li, Y., De Obaldia, M.E., et al., Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells, Cell Stem Cell, 2011, vol. 9, no. 6, pp. 541–552.
Chhabra, A., Lechner, A.J., Ueno, M., et al., Trophoblasts regulate the placental hematopoietic niche through PDGF-B signaling, Dev. Cell, 2012, vol. 22, no. 3, pp. 651–659.
Choi, K., Kennedy, M., Kazarov, A., et al., A common precursor for hematopoietic and endothelial cells, Development, 1998, vol. 125, no. 4, pp. 725–732.
Chou, S. and Lodish, H.F., Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells, Proc. Natl. Acad. Sci. U.S.A., 2010, vol. 107, no. 17, pp. 7799–7804.
Clapp, D.W., Freie, B., Lee, W.H., and Zhang, Y.Y., Molecular evidence that in situ-transduced fetal liver hematopoietic stem/progenitor cells give rise to medullary hematopoiesis in adult, Blood, 1995, vol. 86, pp. 2113–2122.
Cumano, A., Dieterlen-Lièvre, F., and Godin, I., Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura, Cell, 1996, vol. 86, no. 6, pp. 907–916.
Dagher, R.N., Hiatt, K., Traycoff, C., et al., c-Kit and CD38 are expressed by long-term reconstituting hematopoietic cells present in the murine yolk sac, Biol. Blood Marrow Transplant., 1998, vol. 4, no. 2, pp. 69–74.
de Bruijn, M.F., Speck, N.A., Peeters, M.C., and Dzierzak, E., Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo, EMBO J., 2000, vol. 19, no. 11, pp. 2465–2474.
Dieterlen-Lièvre, F., On the origin of haemopoietic stem cells in the avian embryo: an experimental approach, J. Embryol. Exp. Morphol., 1975, vol. 33, no. 3, pp. 607–619.
Dzierzak, E. and Robin, C., Placenta as a source of hematopoietic stem cells, Trends Mol. Biol., 2010, vol. 16, no. 8, pp. 361–367.
Fang, J.S., Gritz, E.C., Marcelo, K.L., and Hirschi, K.K., Isolation of murine embryonic hemogenic endothelial cells, J. Visualized Exp., 2016, vol. 112. https://doi.org/10.3791/54150
Frame, J.M., Fegan, K.H., Conway, S.J., et al., Definitive hematopoiesis in the yolk sac emerges from Wnt-responsive hemogenic endothelium independently of circulation and arterial identity, Stem Cells, 2016, vol. 34, no. 2, pp. 431–444.
Gekas, C., Dieterlen-Lièvre, F., Orkin, S.H., and Mikkola, H.K., The placenta is a niche for hematopoietic stem cells, Dev. Cell, 2005, vol. 8, no. 3, pp. 365–375.
Gekas, C., Rhodes, K.E., van Handel, B., et al., Hematopoietic stem cell development in the placenta, Int. J. Dev. Biol., 2010, vol. 54, nos. 6–7, pp. 1089–1098.
Ginhoux, F., Greter, M., Leboeuf, M., et al., Fate mapping analysis reveals that adult microglia derive from primitive macrophages, Science, 2010, vol. 330, no. 6005, pp. 841–845.
Gomez Perdiguero, E., Klapproth, K., Schulz, C., et al., Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors, Nature, 2015, vol. 518, no. 7540, pp. 547–551.
Haar, J.L. and Ackerman, G.A, A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse, Anat. Rec., 1971, vol. 170, no. 2, pp. 199–223.
He, W.-Y., Lan, Y., Yao, H.-.Y., et al., Interleukin-3 promotes hemangioblast development in mouse aorta-gonad-mesonephros region, Haematologica, 2010, vol. 95, no. 6, pp. 875–883.
Houssaint, E., Differentiation of the mouse hepatic primordium. II. Extrinsic origin of the haemopoietic cell line, Cell Differ., 1981, vol. 10, no. 5, pp. 243–252.
Huber, T.L., Kouskoff, V., Fehling, H.J., et al., Haemangioblast commitment is initiated in the primitive streak of the mouse embryo, Nature, 2004, vol. 432, no. 7017, pp. 625–630.
Ivanovs, A., Rybtsov, S., Welch, L., et al., Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region, J. Exp. Med., 2011, vol. 208, no. 12, pp. 2417–2427.
Ivanovs, A., Rybtsov, S., Anderson, R.A., et al., Identification of the niche and phenotype of the first human hematopoietic stem cells, Stem Cell Rep., 2014, vol. 2, no. 4, pp. 449–456.
Iwasaki, H., Arai, F., Kubota, Y., et al., Endothelial protein C receptor-expressing hematopoietic stem cells reside in the perisinusoidal niche in fetal liver, Blood, 2010, vol. 116, no. 4, pp. 544–553.
Ji, H., Yu, X.Z., and Wagner, T.E., A long-term culture system for the expansion of hematopoietic stem cells from embryonic yolk sac with the capacity to seed erythroid and lymphoid development in vitro and to reconstitute the lymphoid compartment in severe combined immunodeficient mice, Artif. Organs, 1996, vol. 20, no. 10, pp. 1093–1109.
Johnson, G.R. and Moore, M.A., Role of stem cell migration in initiation of mouse fetal liver haemopoiesis, Nature, 1975, vol. 258, no. 5537, pp. 726–728.
Kajikhina, K., Tsuneto, M., and Melchers, F., Environments of hematopoiesis and B-lymphopoiesis in fetal liver, Clin. Exp. Rheumatol., 2015, vol. 33, no. 4, pp. S91–S93.
Kieusseian, A., Brunet de la Grange, P., Burlen-Defranoux, O., et al., Immature hematopoietic stem cells undergo maturation in the fetal liver, Development, 2012, vol. 139, no. 19, pp. 3521–3530.
Kinoshita, T., Sekiguchi, T., Xu, M.-J., et al., Hepatic differentiation induced by oncostatin M attenuates fetal liver hematopoiesis, Proc. Natl. Acad. Sci. U.S.A., 1999, vol. 96, pp. 7265–7270.
Kitajima, K., Kojima, M., Nakajima, K., et al., Definitive but not primitive hematopoiesis is impaired in jumonji mutant mice, Blood, 1999, vol. 93, no. 1, pp. 87–95.
Kumaravelu, P., Hook, L., Morrison, A.M., et al., Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonization of the mouse embryonic liver, Development, 2002, vol. 129, no. 21, pp. 4891–4899.
Lancrin, C., Sroczynska, P., Stephenson, C., et al., The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage, Nature, 2009, vol. 457, no. 723, pp. 892–895.
Liakhovitskaia, A., Gribi, R., Stamateris, E., et al., Restoration of Runx1 expression in the Tie2 cell compartment rescues definitive hematopoietic stem cells and extends life of Runx1 knockout animals until birth, Stem Cells, 2009, vol. 27, no. 7, pp. 1616–1624.
Liakhovitskaia, A., Rybtsov, S., Smith, T., et al., Runx1 is required for progression of CD41+ embryonic precursors into HSCs but not prior to this, Development, 2014, vol. 141, no. 17, pp. 3319–3323.
Liu, C.-P. and Auerbach, R., In vitro development of murine T cells from prethymic and preliver embryonic yolk sac hematopoietic stem cells, Development, 1991, vol. 113, pp. 1315–1323.
Lux, C.T. and Yoder, M.C., Novel methods for determining hematopoietic stem and progenitor cell emergence in the murine yolk sac, Int. J. Dev. Biol., 2010, vol. 54, nos. 6–7, pp. 1003–1009. https://doi.org/10.1387/ijdb.103118cl
Lux, C.T., Yoshimoto, M., McGrath, K., et al., All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac, Blood, 2008, vol. 111, no. 7, pp. 3435–3438.
Medvinsky, A. and Dzierzak, E., Definitive hematopoiesis is autonomously initiated by the AGM region, Cell, 1996, vol. 86, no. 6, pp. 897–906.
Medvinsky, A., Rybtsov, S., and Taoudi, S., Embryonic origin of the adult hematopoietic system: advances and questions, Development, 2011, vol. 138, no. 6, pp. 1017–31.
Migliaccio, G., Migliaccio, A.R., Petti, S., et al., Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac—liver transition, J. Clin. Invest., 1986, vol. 78, no. 1, pp. 51–60.
Moore, M.A.S., Ontogeny of the hematopoietic system, Handb. Stem Cells, 2004, vol. 2, pp. 159–170.
Morel, F., Galy, A., Chen, B., and Szilvassy, S.J., Equal distribution of competitive long-term repopulating stem cells in the CD34+ and CD34– fractions of Thy-1low–Lin–/lowSca-1+ bone marrow cells, Exp. Hematol., 1998, vol. 26, no. 5, pp. 440–448.
Nakano, H., Nakano, H., Liu, X., et al., Hemogenic endocardium contributes to transient definitive hematopoiesis, Nat Commun., 2013, vol. 4, p. 1564. https://doi.org/10.1038/ncomms2569
North, T., Gu, T.L., Stacy, T., et al., Cbfa2 is required for the formation of intra-aortic hematopoietic clusters, Development, 1999, vol. 126, no. 11, pp. 2563–2575.
Oberlin, E., Tavian, M., Blazsek, I., and Péault, B., Blood-forming potential of vascular endothelium in the human embryo, Development, 2002, vol. 129, no. 17, pp. 4147–4157.
Oberlin, E., Fleury, M., Clay, D., et al., VE-cadherin expression allows identification of a new class of hematopoietic stem cells within human embryonic liver, Blood, 2010, vol. 116, no. 22, pp. 4444–4455.
Osawa, M., Nakamura, K., Nishi, N., et al., In vivo self-renewal of c-Kit+Sca-1+Linlow/– hemopoietic stem cells, J. Immunol., 1996, vol. 156, no. 9, pp. 3207–3214.
Ottersbach, K. and Dzierzak, E., The murine placenta contains hematopoietic stem cells within the vascular labyrinth region, Dev. Cell, 2005, vol. 8, no. 3, pp. 377–387.
Padrón-Barthe, L., Temiño, S., Villa del Campo, C., et al., Clonal analysis identifies hemogenic endothelium as the source of the blood-endothelial common lineage in the mouse embryo, Blood, 2014, vol. 124, no. 16, pp. 2523–2532.
Palacios, R. and Imhof, B., At day 8–8.5 of mouse development the yolk sac, not the embryo proper, has lymphoid precursor potential in vivo and in vitro, Proc. Natl. Acad. Sci. U.S.A., 1993, vol. 90, no. 14, pp. 6581–6585.
Palis, J., Robertson, S., Kennedy, M., et al., Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse, Development, 1999, vol. 126, no. 22, pp. 5073–5084.
Palis, J., Chan, R.J., Koniski, A., et al., Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis, Proc. Natl. Acad. Sci. U.S.A., 2001, vol. 98, no. 8, pp. 4528–4533.
Potts, K.S., Sargeant, T.J., Markham, J.F., et al., A lineage of diploid platelet-forming cells precedes polyploid megakaryocyte formation in the mouse embryo, Blood, 2014, vol. 124, no. 17, pp. 2725–2729.
Rampon, C. and Huber, Ph., Multilineage hematopoietic progenitor activity generated autonomously in the mouse yolk sac: analysis using angiogenesis-defective embryos, Int. J. Dev. Biol., 2003, vol. 47, pp. 273–280.
Rhodes, K.E., Gekas, C., Wang, Y., et al., The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation, Cell Stem Cell, 2008, vol. 2, no. 3, pp. 2522–2563.
Richard, C., Drevon, C., Canto, P.Y., et al., Endothelio-mesenchymal interaction controls runx1 expression and modulates the notch pathway to initiate aortic hematopoiesis, Dev. Cell, 2013, vol. 24, no. 6, pp. 600–611.
Robin, C., Ottersbach, K., Durand, C., et al., An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells, Dev. Cell, 2006, vol. 11, no. 2, pp. 171–180.
Robin, C., Bollerot, K., Mendes, S., et al., Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development, Cell Stem Cell, 2009, vol. 5, no. 4, pp. 385–395.
Rybtsov, S., Sobiesiak, M., Taoudi, S., et al., Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region, J. Exp. Med., 2011, vol. 208, no. 6, pp. 1305–1315.
Rybtsov, S., Batsivari, A., Bilotkach, K., et al., Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43– embryonic precursor, Stem Cell Rep., 2014, vol. 3, no. 3, pp. 489–501.
Rybtsov, S., Ivanovs, A., Zhao, S., and Medvinsky, A., Concealed expansion of immature precursors underpins acute burst of adult HSC activity in fetal liver, Development, 2016, vol. 143, no. 8, pp. 1284–1289.
Samokhvalov, I.M., Samokhvalova, N.I., and Nishikawa, S., Cell tracing shows the contribution of the yolk sac to adult haematopoiesis, Nature, 2007, vol. 446, no. 7139, pp. 1056–1061.
Sasaki, K. and Sonoda, Y., Histometrical and three-dimensional analyses of liver hematopoiesis in the mouse embry, Arch. Histol. Cytol., 2000, vol. 63, no. 2, pp. 137–146.
Sasaki, T., Mizuochi, C., Horio, Y., et al., Regulation of hematopoietic cell clusters in the placental niche through SCF/Kit signaling in embryonic mouse, Development, 2010, vol. 137, no. 23, pp. 3941–3952.
Sicurella, C., Freeman, R, Micallef, S., et al., Defective stem cell factor expression in c-myb null fetal liver stroma, Blood Cells, Mol., Dis., 2001, vol. 27, no. 2, pp. 470–478.
Sinka, L., Biasch, K., Khazaal, I., et al., Angiotensin-converting enzyme (CD143) specifies emerging lympho-hematopoietic progenitors in the human embryo, Blood, 2012, vol. 119, no. 16, pp. 3712–3723.
Sonoda, T., Hayashi, C., and Kitamura, Y., Presence of mast cell precursors in the yolk sac of mice, Dev. Biol., 1983, vol. 97, no. 1, pp. 89–94.
Souilhol, C., Gonneau, C., Lendinez, J.G., et al., Inductive interactions mediated by interplay of asymmetric signaling underlie development of adult haematopoietic stem cells, Nat. Commun., 2016, vol. 7, no. 10784. https://doi.org/10.1038/ncomms10784
Sugiyama, D., Inoue-Yokoo, T., Fraser, S.T., et al., Embryonic regulation of the mouse hematopoietic niche, Sci. World J., 2011, vol. 11, pp. 1770–1780.
Tanaka, Y., Sanchez, V., Takata, N., et al., Circulation-independent differentiation pathway from extraembryonic mesoderm toward hematopoietic stem cells via hemogenic angioblasts, Cell Rep., 2014, vol. 8, no. 1, pp. 31–39.
Taoudi, S. and Medvinsky, A., Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta, Proc. Natl. Acad. Sci. U.S.A., 2007, vol. 104, no. 22, pp. 9399–9403.
Taoudi, S., Morrison, A.M., Inoue, H., et al., Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the fetal liver, Development, 2005, vol. 132, no. 18, pp. 4179–4191.
Tavian, M. and Péault, B., The changing cellular environments of hematopoiesis in human development in utero, Exp. Hematol., 2005, vol. 33, no. 9, pp. 1062–1069.
Tavian, M., Hallais, M.-F., and Péault, B., Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo, Development, 1999, vol. 126, no. 4, pp. 793–803.
Tavian, M., Biasch, K., Sinka, L., et al., Embryonic origin of human hematopoiesis, Int. J. Dev. Biol., 2010, vol. 54, nos. 6–7, pp. 1061–1065.
Toles J.F., Chui, D.H., Belbeck, L.W., et al., Hemopoietic stem cells in murine embryonic yolk sac and peripheral blood, Proc. Natl. Acad. Sci. U.S.A., 1989, vol. 86, no. 19, pp. 7456–7459.
Uchida, N., Fujisaki, T., Eaves, A.C., and Eaves, C.J., Transplantable hematopoietic stem cells in human fetal liver have a CD34+ side population (SP) phenotype, J. Clin. Invest., 2001, vol. 108, no. 7, pp. 1071–1077.
Yoder, M.C., Hiatt, K., and Mukherjee, P., In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus, Proc. Natl. Acad. Sci. U.S.A., 1997, vol. 94, no. 13, pp. 6776–6780.
Yokomizo, T. and Dzierzak, E., Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos, Development, 2010, vol. 137, no. 21, pp. 3651–3661.
Zeigler, B.M., Sugiyama, D., Chen, M., et al., The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential, Development, 2006, vol. 133, no. 21, pp. 4183–4192.
Zhou, F., Li, X., Wang, W., et al., Tracing haematopoietic stem cell formation at single-cell resolution, Nature, 2016, vol. 533, no. 7604, pp. 4874–4892.
Funding
The work was performed in the framework of the theme of the state program of basic scientific research of the Institute of Developemental Biology, Russian Academy of Sciences, no. 0108-20186-00045.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by I. Shipounova
Rights and permissions
About this article
Cite this article
Domaratskaya, E.I., Payushina, O.V. Origin of Hematopoietic Stem Cells in Embryonic Development. Biol Bull Rev 9, 191–202 (2019). https://doi.org/10.1134/S2079086419030034
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086419030034