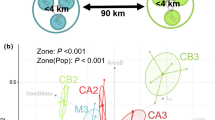
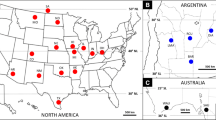
Abstract
Assessing global variation in phenotypic traits and linking that variation to colonization and range expansion is notably rare in invasion biology. Here, we studied variation in seed size in Centaurea solstitialis, a weed with worldwide distribution. Additionally, we explored how seed size variation affects seedling survival of C. solstitialis under favorable precipitation conditions in Anatolia, an ancestral region, and unfavorable precipitation conditions in Argentina, a non-native region. To that end, we conducted seed collections following dispersal pathways of C. solstitialis in ancestral, expanded, and non-native ranges. Locally, collections followed elevation gradients. Also, we performed a greenhouse experiment with C. solstitialis populations varying in seed size and water additions simulating precipitation patterns in Anatolia and Argentina. Seeds from ancestral populations at low elevation were smaller than those from the rest of study populations. Also, seed size in populations at high elevation in the expanded range, the main source of non-native populations, was similar to that in all, but one non-native population, where seeds exhibited further increase. Increments in seed size thus track range expansion in C. solstitialis. Locally, seed size increased with elevation in all three ranges, suggesting convergent responses to that gradient. Seedlings from larger seeds displayed greater survival than those from smaller seeds only under Argentinean conditions. Consequently, populations with large seeds may have been instrumental for colonizing that non-native region. Our findings suggest that ancient and recent dispersal of large-seeded populations contribute to explain the reported global pattern of seed size divergence and worldwide distribution of C. solstitialis.





Similar content being viewed by others
References
Alexander JM, Edwards PJ, Poll M, Parks CG, Dietz H (2009) Establishment of parallel altitudinal clines in traits of native and introduced forbs. Ecology 90:612–622
Baker HG (1972) Seed weight in relation to environmental conditions in California. Ecology 53:997–1010
Barker BS, Andonian K, Swope SM, Luster DG, Dlugosch K (2017) Population genomic analyses reveal a history of range expansion and trait evolution across the native and invaded range of yellow starthistle (Centaurea solstitialis). Mol Ecol 26:1131–1147
Baskin CC, Baskin JM (2014) Seeds: ecology, biogeography and evolution of dormancy and germination, 2nd edn. Academic Press, New York
Benefield CB, DiTomaso JM, Kyser GB (2001) Reproductive biology of yellow starthistle: maximizing late-season control. Weed Sci 49:83–90
Blanquart F, Kaltz O, Nuismer SL, Gandon S (2013) A practical guide to measuring local adaptation. Ecol Lett 16:1195–1205
Blionis GJ, Vokou D (2002) Structural and functional divergence of Campanula spatulata subspecies on Mt. Olympus (Greece). Plant Syst Evol 232:89–105
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHM, White J-SS (2008) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bossdorf O, Auge H, Lafuna L, William ER, Siemann E, Prati D (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11
Bu HY, Chen X, Xu X, Liu K, Jia P, Du G et al (2007) Seed mass and germination in an alpine meadow on the eastern Tsinghai-Tibet plateau. Plant Ecol 191:127–149
Buckley YM, Downey P, Fowler SV, Hill R, Memmott J, Norambuena H, Pitcairn M, Shaw R, Sheppard AW, Winks C, Wittenberg R, Rees M (2003) Are invasives bigger? A global study of seed size variation in two invasive shrubs. Ecology 84:1434–1440
Caddick LR, Linder HP (2002) Evolutionary strategies for reproduction and dispersal in African Restionaceae. Aust J Bot 50:339–355
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Diversity Distrib 15:22–40
Colautti RI, Lau JA (2015) Contemporary evolution during invasion: evidence for differentiation, natural selection, and local adaptation. Mol Ecol 24:1999–2017
Conner JK, Hartl DL (2004) A primer of ecological genetics. Sinauer Associates, Sunderland
Cook RD (1977) Detection of influential observations in linear regression. Technometrics 19:15–18
Cottingham KL, Lennon JT, Brown BL (2005) Knowing when to draw the line: designing more informative ecological experiments. Front Ecol Environ 3:145–152
Crawley MJ (2005) Statistics: an introduction using R. Wiley, Chichester
Dlugosch KM, Cang FA, Barker BS, Andonian K, Swope SM, Rieseberg LH (2015) Evolution of invasiveness through increased resource use in a vacant niche. Nat Plants 1:1–5
Eriksen RL, Hierro JL, Eren Ö, Andonian K, Török K, Becerra PI, Montesinos D, Khetsuriani L, Diaconu A, Kesseli R (2014) Dispersal pathways and genetic differentiation among worldwide populations of the invasive weed Centaurea solstitialis L. (Asteraceae). PLoS One 9:e114786
Gerlach JD (1997) How the west was lost: reconstructing the invasion dynamics of yellow starthistle and other plant invaders of Western rangelands and natural areas. In: Proceedings of the Californian Exotic Pest Plant Council Symposium, vol 3, pp 67–72
Graebner R, Callaway RM, Montesinos D (2012) Invasive species grows faster, competes better, and shows greater evolution toward increased seed size and growth than exotic non-invasive congeners. Plant Ecol 213:545–553
Guo H, Mazer SJ, Du G (2010) Geographic variation in seed mass within and among nine species of Pedicularis (Orobanchaceae): effects of elevation, plant size and seed number per fruit. J Ecol 98:1232–1242
Hierro JL, Villarreal D, Eren Ö, Graham J, Callaway RM (2006) Disturbance facilitates invasions: the effects are stronger abroad than at home. Am Nat 168:144–156
Hierro JL, Eren Ö, Khetsuriani L, Diaconu A, Török K, Montesinos D, Andonian K, Kikodze D, Janoian L, Villarreal D, Estanga-Mollica ME, Callaway RM (2009) Germination responses of an invasive species in native and non-native ranges. Oikos 118:529–538
Hierro JL, Eren Ö, Villarreal D, Chiuffo M (2013) Non-native conditions favor non-native populations of invasive plant: demographic consequences of seed size variation? Oikos 122:583–590
Hijano E, Basigalup D (1995) El cultivo de la alfalfa en la República Argentina. In: Hijano E, Navarro A (eds) La alfalfa en la Argentina. Instituto Nacional de Tecnología Agropecuaria, Buenos Aires, pp 13–18 In Spanish
Hodgins KA, Bock DG, Rieseberg LH (2018) Trait evolution in invasive species. Annu Plant Rev 1:1–37
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Syst 13:201–228
Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L (2000) Rapid evolution of a geographic cline in size in an introduced fly. Science 287:308–310
Joley DB, Maddox DM, Schoenig SE, Mackey BE (2003) Parameters affecting germinability and seed bank dynamics in dimorphic achenes of Centaurea solstitialis in California. Can J Bot 81:993–1007
Kawecki TJ (2008) Adaptation to marginal habitats. Ann Rev Ecol Syst 39:321–342
Leger EA, Rice KJ (2007) Assessing the speed and predictability of local adaptation in invasive California poppies (Eschscholzia californica). J Evol Biol 20:1090–1103
Leishman MR, Westoby M (1994) The role of seed size in seedling establishment in dry soil conditions—experimental evidence from semi-arid species. J Ecol 82:249–258
Leishman MR, Wright IJ, Moles A, Westoby M (2000) The evolutionary ecology of seed size. In: Fenner M (ed) Seeds, the ecology of regeneration in plant communities, 2nd edn. CABI Publishing, New York, pp 31–58
Lomolino MV, Riddle BR, Whittaker RJ (2017) Biogeography, 5th edn. Oxford University Press, Sunderland
MacDougall AS, McCune JL, Eriksson O, Cousins SAO, Pärtel M, Firn J, Hierro JL (2018) The neolithic plant invasion hypothesis: the role of preadaptation and disturbance in grassland invasion. New Phytol 220:94–103
Maddox DM, Mayfield A, Poritz NH (1985) Distribution of yellow starthistle (Centaurea solstitialis) and Russian knapweed (Centaurea repens). Weed Sci 33:315–327
Maron JL, Vilà M, Bommarco R, Elmendorf S, Beardsley P (2004) Rapid evolution of an invasive plant. Ecol Monog 74:261–280
Maron JL, Elmendorf SC, Vilà M (2007) Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum. Evolution 61:1912–1924
Mazer SJ (1989) Ecological, taxonomic and life history correlates of seed mass among Indiana dune angiosperms. Ecol Monog 59:153–175
Miguel MF, Lortie CJ, Callaway RM, Hierro JL (2017) Competition does not come at the expense of colonization in seed morphs with increased size and germination. Am J Bot 104:1323–1333
Moles AT, Westoby M (2004) Seedling survival and seed size: a synthesis of the literature. J Ecol 92:372–383
Moles AT, Westoby M (2006) Seed size and plant strategy across the whole life cycle. Oikos 113:91–105
Montesinos D, Callaway RM (2017) Inter-regional hybrids of native and invasive Centaurea solstitialis display intermediate competitive ability. Ecography 40:801–802
Montesinos D, Santiago G, Callaway RM (2012) Neo-allopatry and rapid reproductive isolation. Am Nat 180:529–533
Pausas JG, Bond WJ (2019) Humboldt and the reinvention of nature. J Ecol 107:1031–1037
Pearson DE, Ortega YK, Eren Ö, Hierro JL (2018) Community assembly theory as a framework for biological invasions. Trends Ecol Evol 33:313–325
Peco B, Rico L, Azcárate FM (2009) Seed size and response to rainfall patterns in annual grasslands: 16 years of permanent plot data. J Veg Sci 20:8–16
Pluess AR, Schutz W, Stöcklin J (2005) Seed weight increases with altitude in the Swiss Alps between related species but not among populations of individual species. Oecologia 144:55–61
Qi W, Bu H, Cornelissen JHC, Zhang C, Guo S, Wang J, Zhou X, Li W, Du G (2015) Untangling interacting mechanisms of seed mass variation with elevation: insights from the comparison of inter-specific and intra-specific studies on eastern Tibetan angiosperm. Plant Ecol 216:283–292
Rees M, Westoby M (1997) Game-theoretical evolution of seed mass in multi-species ecological models. Oikos 78:116–126
Sheley RL, Larson LL (1994) Observation: comparative life history of cheatgrass and yellow starthistle. J Range Manag 47:450–456
Suárez-Vidal E, Sampedro L, Zas R (2017) Is the benefit of larger seed provisioning on seedling performance greater under abiotic stress? Environ Exp Bot 134:45–53
Sun M, Ritland K (1998) Mating system of yellow starthistle (Centaurea solstitialis), a successful colonizer in North America. Heredity 80:225–232
Thompson KA, Renaudin M, Johnson MTJ (2016) Urbanization drives the evolution of parallel clines in plant populations. Proc R Soc B 283:20162180
Timson J (1965) New method of recording germination data. Nature 207:216–217
Totland Ø, Birks HJB (1996) Factors influencing interpopulation variation in Ranunculus acris seed production in an alpine area of southwestern Norway. Ecography 19:269–278
van Kleunen M, Bossdorf O, Dawson W (2018) The ecology and evolution of alien plants. Annu Rev Ecol Evol Syst 49:25–47
Venable DL, Brown JS (1988) The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. Am Nat 131:360–384
Vera ML (1997) Effects of altitude and seed size on germination and seedling survival of heathland plants in north Spain. Plant Ecol 133:101–106
Vitousek PM, D’Antonio CM, Loope LL, Rejmánek Westbrooks R (1997) Introduced species: a significant component of human-cause global change. N Z J Ecol 21:1–16
Westoby M, Jurado E, Leishman M (1992) Comparative evolutionary ecology of seed size. Trends Ecol Evol 7:368–372
Widmer TL, Guermache F, Dolgovskaia MY, Reznik SY (2007) Enhanced growth and seed properties in introduced vs. native populations of yellow starthistle (Centaurea solstitialis). Weed Sci 55:465–473
Willis SG, Hulme PE (2004) Environmental severity and variation in the reproductive traits of Impatiens glandulifera. Funct Ecol 18:887–898
Acknowledgements
We thank M.N. Doĝan for generously facilitating greenhouse space, M. Şenturk, L. Ramirez Brumatti, N.B. Ramirez Haberkon, N. Loyola, and A. Parras for field, lab, and greenhouse assistance, G. Vergara for rainfall data from Santa Rosa, and J. Lembrechts and an anonymous reviewer for critically commenting an earlier version of this manuscript. Margherita Gioria provided major insights to our study. This project was partially funded by CONICET (Abroad Fellowship for Young Researchers) and UNLPam (CN219).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hierro, J.L., Eren, Ö., Montesinos, D. et al. Increments in weed seed size track global range expansion and contribute to colonization in a non-native region. Biol Invasions 22, 969–982 (2020). https://doi.org/10.1007/s10530-019-02137-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-019-02137-z