Abstract
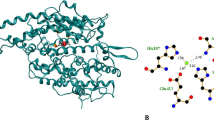
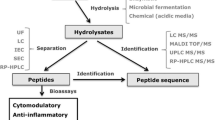
Hypertension is nowadays one of the major world concerns in public health. Several food proteins, among which caseins, can be substrates for generating peptides with antihypertensive potential. With the increasingly evolution of computational tools, in silico molecular modeling have gained prominence in studies of protein-ligand complexes in different research fields, such as pharmaceutics and biochemical engineering. However, the application of such methodologies in food-related research can be considered still embryonic. Thus, the central aim of the present work was to apply molecular modelling in order to elucidate the molecular bases of the anti-hypertensive potential of milk caseins-derived peptides. Firstly, hydrolysates obtained from a controlled trypsinolysis of caseins were fractioned according to their molecular weight, by ultrafiltration and RP-HPLC. The obtained fractions were evaluated with regard to their in vitro inhibitory angiotensin-converting enzyme activity (%IACE). Six chromatographic fractions were identified, and three of them displayed high ACE-inhibition (F1: 80.68%; F2: 79.00%; and F4: 62.44%). Finally, intermolecular interactions networks in complexes formed between ACE and the identified peptides were assessed through in silico molecular docking. At the molecular level, a correlation between in vitro and in silico results was found: the peptides FFVAPFPEVFGK (F6), FALPQYLK (F2, F4) and ALNEINQFYQK (F1) presented the lowest biding energies and interacted by specific H-bonds, electrostatic and hydrophobic interactions formed within ACE active site S1 residues (Ala354, Glu384, and Tyr 523) and the Zn2+ coordinated residues (His383, His387, and Glu411). The fraction F3, despite its low total peptide concentration, presented a moderate inhibitory activity for ACE (49.2%), likely due to H-bonds between HQGLPQEVLNENLLR and the active site S1 residues.



Similar content being viewed by others
References
World Health Organization (WHO): http://www.fao.org/3/AC911E/AC911E00.htm. Accessed 25 Jan 2019
J. Torruco-Uco, L. Chel-Guerrerob, A. Martínez-Ayala, G. Dávila-Ortíz, D. Betancur-Ancona, LWT - food Sci. Technol. 42, 1597 (2009)
Z.F. Bhat, S. Kumar, H.F. Bhat, Crit. Rev. Food Sci. Nutr. 57, 566 (2015)
M.R. De Oliveira, T.J. Silva, E. Barros, V.M. Guimarães, M.C. Baracat-Pereira, M.R. Eller, J.S. dos Coimbra, E.B. De Oliveira, Appl. Biochem. Biotechnol. 185, 884 (2018)
E.C. de Souza Jr, J.S. dos Coimbra, E.B. de Oliveira, R.C.F. Bonomo, J. Chromatogr. B 973, 84 (2014)
F.G. Amorim, L.B. Coitinho, A.T. Dias, A.G.F. Friques, B.L. Monteiro, L.C.D. de Rezende, T.M.C. Pereira, B.P. Campagnaro, E. De Pauw, E.C. Vasquez, L. Quinton, Food Chem. 282, 109 (2019)
M. Tu, C. Wang, C. Chen, R. Zhang, H. Liu, W. Lu, Food Chem. 256, 98 (2018)
R.J.S. Castro, H.H. Sato, Food Res. Int. 74, 185 (2015)
X. Lan, D. Liao, S. Wu, F. Wang, J. Sun, Z. Tong, Food Chem. 182, 136 (2015)
O. Abdelhedi, R. Nasri, L. Mora, M. Jridi, F. Toldra, M. Nasri, Food Chem. 239, 453 (2018)
M. Mirzaei, S. Mirdamadi, R.M. Ehsani, J. Food Drug Anal. 26, 696 (2018)
A. Shi, H. Liu, L. Liu, H. Hu, Q. Wang, B. Adhikari, PLoS One 9, 23 (2014)
T.M. Menezes, S.M.V. de Almeida, R.O. de Moura, G. Seabra, C.A. de Lima, J.L. Neves, Int. J. Biol. Macromol 122, 289 (2019)
D.B. Kitchen, H. Decornez, J.R. Furr, J. Bajorath, Nat. Rev. 3, 935 (2004)
P. García-mora, M. Martín-martínez, M.A. Bonache, M. Angeles, R. González-múniz, E. Peñas, J. Frias, C. Martinez-villaluenga, Food Chem. 221, 464 (2017)
X. Wang, H. Chen, X. Fu, S. Li, J. Wei, LWT - food Sci. Technol. 75, 93 (2017)
K. Lin, L. Zhang, X. Han, D. Cheng, J. Funct. Foods 32, 266 (2017)
S.C. Cheison, J. Brand, E. Leeb, U. Kulozik, Agric. Food Chem. 59, 1572 (2011)
G.W. Hofland, M. Van Es, L.A.M. Van Der Wielen, G. Witkamp, Ind. Eng. Chem. Res. 38, 4919 (1999)
D.W. Cushman, H.S. Cheung, Biochem. Pharmacol. 20, 1637 (1971)
A. Keller, A. I. Nesvizhskii, E. Kolker, and R. Aebersold, 74, 5383 (2002)
R. Natesh, S. L. U. Schwager, E. D. Sturrock, and K. R. Acharya, 421, 551 (2003)
J.M. Abraham, T. Murtola, R. Schulz, S. Pall, J.C. Smith, B. Hess, E. Lindahl, SoftwareX 1–2, 1 (2015)
C. Oostenbrink, A. Villa, A.E. Mark, W.F. Van Gunsteren, J. Comput. Chem. 25, 1656 (2004)
H.J.C. Berendsen, J.R. Grigera, T.P. Straatsma, J. Phys. Chem. 91, 6269 (1987)
E.B. De Oliveira, C. Humeau, L. Chebil, E.R. Maia, F. Dehez, B. Maigret, M. Ghoul, J.-M. Engasser, J. Mol. Catal. B Enzym. 59, 96 (2009)
M.D. Polêto, M.P. Alves, R. Ligabue-braun, M.R. Eller, A.F. de Carvalho, Food Chem. 286, 309 (2019)
O. Trott, A.J. Olson, Wiley Period. 31, 455 (2010)
E.C. de Souza, J.S.D.R. Coimbra, E.B. de Oliveira, R.C.F. Bonomo, J. Chromatogr, B. Analyt. Technol. Biomed. Life Sci. 973C, 84 (2014)
A. Yamada, T. Sakurai, D. Ochi, E. Mitsuyama, K. Yamauchi, F. Abe, Food Chem. 172, 441 (2015)
R.J. Bazan, J.F. Fletterick, Virology 171, 637 (1989)
T. Aoki, Y. Kako, T. Imamura, J. Dairy Res. 53, 53 (1986)
J. Kyte, J. Doolittle, Mol. Biol. 157, 105 (1982)
Q. Wu, J. Jia, H. Yan, J. Du, Z. Gui, Peptides 68, 17 (2015)
A.S. Pina, A.C.A. Roque, J. Mol. Recognit. 22, 162 (2009)
S. Maruyama, H. Mitachi, H. Tanaka, N. Tomizuka, H. Suzuki, Agric. Biol. Chem. 51, 1581 (1987)
I. López-Expósito, A. Quirós, L. Amigo, I. Recio, Lait 87, 241 (2007)
J. Tauzin, L. Miclo, J. Gaillard, FEBS Lett. 531, 4 (2002)
R.J. Fitzgerald, B.A. Murray, D.J. Walsh, J. Nutr. 134, 980S (2004)
M. Abdel-hamid, J. Otte, C. De Gobba, A. Osman, Int. Dairy J. 66, 91 (2017)
A.T. Girgih, R. He, R.E. Aluko, J. Agric. Food Chem. 62, 4135 (2014)
P. Li, J. Jia, M. Fang, L. Zhang, M. Guo, J. Xie, Process Biochem. 49, 898 (2014)
J. Tauzin, L. Miclo, G. Jean-luc, FEBS Lett. 531, 4 (2002)
Acknowledgements
We are grateful to BIOAGRO-UFV, NuBioMol-UFV and UFV Computational Cluster for providing the facilities for carrying out the experiments, and to Brazilian agencies CAPES, CNPq, FAPEMIG, FINEP, FUNARBE and SisNANO/MCTI, for the financial support. Ms. M.R. Oliveira and Ms. T.J. Silva are especially grateful to CNPq for their scholarships.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1619 kb)
Rights and permissions
About this article
Cite this article
De Oliveira, T.V., Polêto, M.D., De Oliveira, M.R. et al. Casein-Derived Peptides with Antihypertensive Potential: Production, Identification and Assessment of Complex Formation with Angiotensin I-Converting Enzyme (ACE) through Molecular Docking Studies. Food Biophysics 15, 162–172 (2020). https://doi.org/10.1007/s11483-019-09616-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-019-09616-9