Abstract
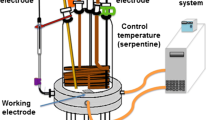
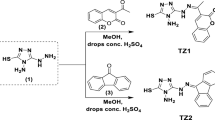
Two newly synthesized ligands based on 1,3,4-thiadiazolethiosemicarbazone have been isolated by the condensation reaction of 2,3-disubstituted-5-acetyl-1,3,4-thiadiazole derivatives with thiosemicarbazide in acidic medium in addition to their Co(II) chelates. The synthesized cobalt chelates that have been obtained by the reaction of each ligand with cobalt acetate were confirmed to have the formulae [(LM)Co(OAc)(H2O)2]H2O (LM–Co) and [(LN)Co(OAc)(H2O)2]0.5CH3OH (LN–Co); where LM and LN are 1,3,4-thiadiazolethiosemicarbazone ligands with methyl and nitro substituents, respectively. Comparison of the IR spectrum of each ligand with that of its cobalt complex implied that both ligands acted as monobasic tridentate connecting to the cobalt ion through N atoms of both azomethine group and thiadiazole ring and S atom of deprotonated SH group as well. The two complexes have been proved to have octahedral geometrical structures. The synthesized compounds were studied as corrosion inhibitors for carbon steel in molar hydrochloric acid solution using several chemical and electrochemical techniques. The investigational outcomes displayed that the inhibition efficiencies of the examined compounds were found to augment as the concentrations of such compounds raised. At comparable inhibitors concentration, the inhibition efficiency was a little increased following the order: LM > LM–Co > LN > LN–Co. The acquired high inhibition efficiencies of the explored compounds were ascribed to the potent adsorption of the molecules on the steel surface and construction of adherent layers. Such adsorption was found to accord with Langmuir adsorption isotherm. There is a good correlation in the results obtained from the different measurements used.








Similar content being viewed by others
References
D. Karcz, A. Matwijczuk, B. Boron, B. Creaven, L. Fiedor, A. Niewiadomy, M. Gagos, Isolation and spectroscopic characterization of Zn(II), Cu(II), and Pd(II) complexes of 1,3,4-thiadiazole-derived ligand. J. Mol. Struct. 1128, 44–50 (2017)
L.M.T. Frija, A.J.L. Pombeiro, M.N. Kopylovich, Coordination chemistry of thiazoles, isothiazoles and thiadiazoles. Coord. Chem. Rev. 308, 32–55 (2016)
K. Zhang, H. Zheng, C. Hua, M. Xin, J. Gao, Y. Li, Novel fluorescent N,O-chelated fluorine-boron benzamide complexes containing thiadiazoles: synthesis and fluorescence characteristics. Tetrahedron 74, 4161–4167 (2018)
C. Richardson, P.J. Steel, D.M. D’Alessandro, P.C. Junk, F.R. Keene, Mono- and di-nuclear complexes of the ligands 3,4-di(2-pyridyl)-1,2,5-oxadiazole and 3,4-di(2-pyridyl)-1,2,5-thiadiazole; new bridges allowing unusually strong metal–metal interactions. J. Chem. Soc. Dalton Trans. (2002). https://doi.org/10.1039/B202954E
G.-L. Wen, Y.-Y. Wang, P. Liu, C.-Y. Guo, W.-H. Zhang, Q.-Z. Shi, A series of 1-D to 3-D metal–organic coordination architectures assembled with V-shaped bis(pyridyl)thiadiazole under co-ligand intervention. Inorg. Chim. Acta 362, 1730–1738 (2009)
B. Ardan, Y. Slyvka, E. Goreshnik, M. Myskiv, First N-allyl-aminothiadiazole copper(i) π-complexes: synthesis and structural peculiarities of Cu(L)CF3SO3] and [Cu2(L)2(H2O)2](SiF6) · 2.5H2O compounds (L = 2-(allyl)-amino-5-methyl-1,3,4-thiadiazole). Acta Chim. Slov. 60, 484–490 (2013)
S. Chandra, S. Gautam, A. Kumar, M. Madan, Coordination mode of pentadentate ligand derivative of 5-amino-1,3,4-thiadiazole-2-thiol with nickel(II) and copper(II) metal ions: synthesis, spectroscopic characterization, molecular modeling and fungicidal study. Spectrochim. Acta A 136, 672–681 (2015)
A. Smaili, L.A. Rifai, S. Esserti, T. Koussa, F. Bentiss, S. Guesmi, A. Laachir, M. Faize, Copper complexes of the 1,3,4-thiadiazole derivatives modulate antioxidant defense responses and resistance in tomato plants against fungal and bacterial diseases. Pestic. Biochem. Physiol. 143, 26–32 (2017)
H. Zine, L.A. Rifai, T. Koussa, F. Bentiss, S. Guesmi, A. Laachir, K. Makroum, M. Belfaiza, M. Faize, The mononuclear nickel (II) complex bis(azido-kN)bis[2,5-bis(pyridin-2-yl)-1,3,4-thiadiazole-κ2N2, N3]nickel(II) protects tomato from Verticillium dahliae by inhibiting the fungal growth and activating plant defenses. Pest Manag. Sci. 73, 188–197 (2017)
M. El Azhar, B. Mernari, M. Traisnel, F. Bentiss, M. Lagrenée, Corrosion inhibition of mild steel by the new class of inhibitors [2,5-bis(n-pyridyl)-1,3,4-thiadiazoles] in acidic media. Corrosion sci. 43, 2229–2238 (2001)
F. Bentiss, M. Lebrini, H. Vezin, M. Lagrené, Experimental and theoretical study of 3-pyridyl-substituted 1,2,4-thiadiazole and 1,3,4-thiadiazole as corrosion inhibitors of mild steel in acidic media. Mater. Chem. Phys. 87, 18–23 (2004)
F. Bentiss, M. Traisnel, M. Lagrenee, Influence of 2,5-bis(4-dimethylaminophenyl)-1,3,4-thiadiazole on corrosion inhibition of mild steel in acidic media. J. Appl. Electrochem. 31, 41–48 (2001)
M.A. Arenos, M. Bethencourt, F.G. Botana, J. Domborenena, M. Marcos, Inhibition of 5083 aluminium alloy and galvanised steel by lanthanide salts. Corros. Sci. 43, 157–170 (2001)
D. Gustincic, A. Kokalj, DFT study of azole corrosion inhibitors on Cu2O model of oxidized copper surfaces: I. Molecule–surface and Cl–surface bonding. Metals 8, 311–338 (2018)
I.A. Arkhipushkin, K.S. Shikhaliev, A.Y. Potapov, L.V. Sapronova, L.P. Kazansky, Inhibition of brass (80/20) by 5-mercaptopentyl-3-amino-1,2,4-triazole in neutral solutions. Metals 7, 488–500 (2017)
A. Fawzy, M. Abdallah, I.A. Zaafarany, S.A. Ahmed, I.I. Althagafi, Thermodynamic, kinetic and mechanistic approach to the corrosion inhibition of carbon steel by new synthesized amino acids-based surfactants as green inhibitors in neutral and alkaline aqueous media. J. Mol. Liq. 265, 276–291 (2018)
A. Fawzy, I.A. Zaafarany, H.M. Ali, M. Abdallah, Corrosion inhibition performance of a novel cationic surfactant for protection of carbon steel pipeline in acidic media. Int. J. Electrochem. Sci. 13, 4575–6842 (2018)
R.F. Godec, M.G. Pavlovic, Synergistic effect between non-ionic surfactant and halide ions in the forms of inorganic or organic salts for the corrosion inhibition of stainless-steel X4Cr13 in sulphuric acid. Corros. Sci. 58, 192–201 (2012)
E. Khamis, M.A. Ameer, N.M. AlAndis, G. Al-Senani, Effect of thiosemicarbazones on corrosion of steel in phosphoric acid produced by wet process. Corrosion 56, 127–138 (2000)
N. Karakus, K. Sayin, The investigation of corrosion inhibition efficiency on some benzaldehyde thiosemicarbazones and their thiole tautomers: computational study. J. Taiwan Inst. Chem. E. 48, 95–102 (2015)
C. Verma, L.O. Olasunkanmi, E.E. Ebenso, M.A. Quraishi, Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: a review. J. Mol. Liq. 251, 100–118 (2018)
M. Yadav, S. Kumar, I. Bahadur, D. Ramjugernath, Electrochemical and quantum chemical studies on synthesized phenylazopyrimidone dyes as corrosion inhibitors for mild steel in a 15% HCl solution. Int. J. Electrochem. Sci. 9, 3928–3950 (2014)
M.A. Hegazy, H.M. Ahmed, A.S. El-Tabei, Investigation of the inhibitive effect of p-substituted 4-(N,N,N-dimethyldodecylammonium bromide)benzylidene-benzene-2-yl-amine on corrosion of carbon steel pipelines in acidic medium. Corros. Sci. 53, 671–678 (2011)
S.K. Saha, A. Dutta, P. Ghosh, D. Sukul, P. Banerjee, Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: experimental and theoretical approach. Phys. Chem. Chem. Phys. 18, 17898–17911 (2016)
N.F. Eweiss, A.O. Osman, Synthesis of heterocycles. Part II. New routes to acetylthiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 17, 1713–1717 (1980)
W.J. Geary, The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 7, 81–122 (1971)
H. El-Ghamry, N. El-Wakiel, A. Khamis, Synthesis, structure, antiproliferative activity and molecular docking of divalent and trivalent metal complexes of 4H‐3,5‐diamino‐1,2,4‐triazole and α‐hydroxynaphthaldehyde Schiff base ligand. Appl. Organomet. Chem. 32, e4583 (2018)
M.M. Alsharekh, I.I. Althagafi, M.R. Shaaban, T.A. Farghaly, Microwave-assisted and thermal synthesis of nanosized thiazolyl-phenothiazine derivatives and their biological activities. Res. Chem. Intermed. 45, 127–154 (2019)
H.A. El-Ghamry, M. Gaber, T.A. Farghaly, Synthesis, structural characterization, molecular modeling and DNA binding ability of CoII, NiII, CuII, ZnII, PdII and CdII complexes of benzocycloheptenone thiosemicarbazone ligand. Mini. Rev. Med. Chem. 19, 1068–1079 (2019)
H.A. El-Ghamry, K. Sakai, S. Masaoka, K. El-Baradie, R. Issa, Synthesis and characterization of self-assembled coordination polymers of N-diaminomethylene-4-(3-formyl-4-hydroxy-phenylazo)-benzenesulfonamide. J. Coord. Chem. 65, 780–794 (2012)
P.F. Rapheal, E. Manoj, M.R. PrathapachandraKurup, Copper(II) complexes of N(4)-substituted thiosemicarbazones derived from pyridine-2-carbaldehyde: crystal structure of a binuclear complex. Polyhedron 26, 818–828 (2007)
K. Nakamoto (ed.), Infrared spectra of inorganic and coordination compounds (Wiley, New York, 1986)
D.-D. Yang, R. Wang, J.-L. Zhu, Q.-Y. Cao, J. Qin, H.-L. Zhu, Synthesis, crystal structures, molecular docking, in vitro monoamine oxidase-B inhibitory activity of transition metal complexes with 2-{4-[bis (4-fluorophenyl)methyl]piperazin-1-yl} acetic acid. J. Mol. Struct. 1128, 493–498 (2017)
A.M. Gouda, H.A. El-Ghamry, T.M. Bawazeer, T.A. Farghaly, A.N. Abdalla, A. Aslam, Antitumor activity of pyrrolizines and their Cu(II) complexes: design, synthesis and cytotoxic screening with potential apoptosis-inducing activity. Eur. J. Med. Chem. 145, 350–359 (2018)
M. Shakir, A. Abbasi, M. Azam, A.U. Khan, Synthesis, spectroscopic studies and crystal structure of the Schiff base ligand L derived from condensation of 2-thiophenecarboxaldehyde and 3,3′-diaminobenzidine and its complexes with Co(II), Ni(II), Cu(II), Cd(II) and Hg(II): comparative DNA binding studies of L and its Co(II), Ni(II) and Cu(II) complexes. Spectrochim. Acta A 79, 1866–1875 (2011)
N.H. Yarkandi, H.A. El-Ghamry, M. Gaber, Synthesis, spectroscopic and DNA binding ability of CoII, NiII, CuII and ZnII complexes of Schiff base ligand (E)-1-(((1H-benzo[d]imidazol-2-yl)methylimino)methyl)naphthalen-2-ol. X-ray crystal structure determination of cobalt (II) complex. Mater. Sci. Eng., C 75, 1059–1067 (2017)
A.B.P. Lever, Inorganic electronic spectroscopy, 2nd edn. (Elsevier, Amsterdam, 1984)
M. Pfaller, L. Burmeister, M.A. Bartlett, M.G. Rinaldi, Multicenter evaluation of four methods of yeast inoculum preparation. J. Clin. Microbiol. 26, 1437–1441 (1988)
L.B. Tang, G.N. Mu, G.H. Liu, The effect of neutral red on the corrosion inhibition of cold rolled steel in 1.0 M hydrochloric acid. Corros. Sci. 45, 2251–2262 (2003)
P. Manjula, S. Manonmani, P. Jayaram, S. Rajendran, Corrosion behaviour of carbon steel in the presence of N-cetyl-N,N,N-trimethylammonium bromide, Zn2+ and calcium gluconate. Anti-Corros. Methods Mater. 48, 319–324 (2001)
H. Ma, S. Chen, L. Niu, S. Zhao, S. Li, D. Li, Inhibition of copper corrosion by several Schiff bases in aerated halide solutions. J. Appl. Electrochem. 32, 65–72 (2002)
M. Abdallah, H.M. Altass, B.A. Al Jahdaly, M.M. Salem, Some natural aqueous extracts of plants as green inhibitor for dissolution of carbon steel in 0.5 M sulfuric acid. Green Chem. Lett. Rev. 11, 189–196 (2018)
M. Christov, A. Popova, Adsorption characteristics of corrosion inhibitors from corrosion rate measurements. Corros. Sci. 46, 1613–1620 (2004)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bawazeer, T.M., El-Ghamry, H.A., Farghaly, T.A. et al. Novel 1,3,4-Thiadiazolethiosemicarbazones Derivatives and Their Divalent Cobalt-Complexes: Synthesis, Characterization and Their Efficiencies for Acidic Corrosion Inhibition of Carbon Steel. J Inorg Organomet Polym 30, 1609–1620 (2020). https://doi.org/10.1007/s10904-019-01308-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01308-8