Abstract
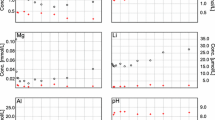
The Manuguru geothermal area, located in the Khammam district of Telangana state, India, is one of the least explored medium-enthalpy geothermal systems in India. In this study, subsurface reservoir temperature was estimated by applying various methodologies such as chemical geothermometry, multicomponent geothermometry and mixing models. Chemical geothermometers provided wide range in temperature estimation, and most of them (Na–K, Na–K–Ca, Mg-corrected Na–K–Ca) were found to be unsuitable for predicting reservoir temperature due to the absence of attainment of equilibrium between suitable mineral pairs. The temperature range estimated from the quartz geothermometers varied from 72 to 120 °C which matched closely with values obtained from K–Mg geothermometers. To overcome this problem and to better constrain the reservoir temperature, multicomponent solute geothermometry modelling was carried out by applying the GeoT computer code. Fluid reconstruction was done after taking into account both the degassing and mixing phenomena. GeoT modelling of the reconstructed fluid provided excellent clustering of the minerals. From the GeoT modelling study, it was found that minerals like quartz, chalcedony, calcite, etc., attained simultaneous equilibrium with thermal waters in the temperature range of 130 ± 10 °C, which can be taken as the most probable reservoir temperature. The subsurface temperature (137 °C) obtained from the mixing model further validated the results obtained from multicomponent geothermometry. This integrated multicomponent method and the simulation program used in this study take into account various processes (i.e. mixing, degassing, non-attainment of equilibrium, etc.) which affect the composition of the thermal fluids during its ascent to the surface. The statistical approach of ‘best clustering minerals’ used in this model helps to overcome the problems encountered in applying cation or single-component geothermometers in the medium-enthalpy geothermal systems.






Similar content being viewed by others
References
APHA (American Public Health Association) (1985) Standard methods for the examination of water and waste water, 6th edn. APHA, Washington, DC
Apollaro C, Dotsika E, Marini L, Barca D, Bloise A, De Rosa R, Doveri M, Lelli M, Muto F (2012) Chemical and isotopic characterization of the thermo mineral water of Terme Sibarite springs (Northern Calabria, Italy). Geochem J 46:117–129
Apollaro C, Vespasiano G, Muto F, De Rosa R, Barca D, Marini L (2016) Use of mean residence time of water, flowrate, and equilibrium temperature indicated by water geothermometers to rank geothermal resources. Application to the thermal water circuits of Northern Calabria. J Volcanol Geotherm Res 328:147–158
Arnorsson S (1983) Chemical equilibria in Icelandic geothermal systems-implications for chemical geothermometry investigations. Geothermics 12:119–128
Arnorsson S (2000) Isotopic and chemical techniques in geothermal exploration, development and use. International Atomic Energy Agency, Vienna, pp 154–170
CGWB (Central Ground Water Board of India) (2013) Ground water brochure. Khammam district, Andhra Pradesh, pp 1–22
Chandrasekharam D, Rao VG, Jayaprakash SJ (1996) Geothermal energy potential and direct use of geothermal springs, Godavari valley, Andhra Pradesh. Geological Survey of India. Special publication, vol 45. Geothermal energy of India, Nagpur, pp 151–161
Chatterjee S, Sharma S, Ansari MA, Deodhar AS, Low U, Sinha UK, Dash A (2016) Characterization of subsurface processes estimation of reservoir temperature in Tural Rajwadi geothermal fields, Maharashtra, India. Geothermics 59:77–89. https://doi.org/10.1016/j.geothermics.2015.10.011
Chatterjee S, Ansari MA, Deodhar AS, Sinha UK, Dash A (2017a) A multi-isotope approach (O, H, C, S, B and Sr) to understand the source of water and solutes in some the thermal springs from West Coast geothermal area, India. Arab J Geosci 10:242. https://doi.org/10.1007/s12517-017-3022-0
Chatterjee S, Sarkar A, Deodhar AS, Biswal BP, Jaryal A, Mohokar HV, Sinha UK, Dash A (2017b) Geochemical and isotope hydrological characterisation of geothermal resources at Godavari valley, India. Environ Earth Sci 76:1–21. https://doi.org/10.1007/s12655-017-6411-5
Chatterjee S, Sinha UK, Ansari MA, Mohokar HV, Dash A (2018) Application of lumped parameter model to estimate mean transit time (MTT) of the thermal water using environmental tracer (3H): Insight from Uttarakhand geothermal area (India). Appl Geochem 94(2018):1–10. https://doi.org/10.1016/j.apgeochem.2018.04.013
Ellis AJ (1979) Chemical geothermometry in geothermal systems. Geothermics 25:219–226
Fournier RO (1977) Chemical geothermometers and mixing models for geothermal systems. Geothermics 5:40–41
Fournier RO (1979) A revised equation for Na/K geothermometer. Trans Geotherm Resour Counc 3:221–224
Fournier RO (1989) The solubility of silica in hydrothermal solutions: practical applications. In: Lectures on geochemical interpretation of hydrothermal water. UNU geothermal training programme, report 10, Raykjavik, Iceland
Fournier RO, Potter RW (1979) Magnesium correction to the Na–K–Ca chemical geothermometer. Geochim Cosmochim Acta 43:1543–1550
Fournier RO, Truesdell AH (1973) An empirical Na–K–Ca geothermometer from natural waters. Geochim Cosmochim Acta 37:1255–1275
Giggenbach WF (1988) Geothermal solute equilibria. Derivation of Na–K–Mg–K geoindicators. Geochim Cosmochim Acta 52:2749–2765
Keenan JH, Keyes FG, Hill PG, Moore JG (1969) Steam tables – thermodynamic properties of water including vapour, liquid and solid phases (international edition – metric units). Wiley, New York, p 162
Nicholson K (1993) Geothermal fluids chemistry and exploration techniques. Springer, Belin, pp 22–23
Nieva D, Nieva R (1987) Developments in geothermal energy in Mexico, part 12. A cationic geothermometer for prospecting of geothermal resources. Heat Recover Syst CHP 7:243–258
Pang ZH, Reed MH (1998) Theoretical chemical thermometry on geothermal waters: problems and methods. Geochim Cosmochim Acta 62:1083–1091
Reed MH, Spycher NF (1984) Calculation of pH and mineral equilibria in hydrothermal waters with application to geothermometry and studies of boiling and dilution. Geochim Cosmochim Acta 48:1479–1492
Santoyo E, Díaz-González L (2010) A new improved proposal of the Na/K geothermometer to estimate deep equilibrium temperatures and their uncertainties in geothermal systems. In: Proceedings world geothermal congress, Bali, Indonesia, 25–29 April
Saxena VK, Gupta ML (1985) Aquifer chemistry of thermal waters of the Godavari valley, India. J Volcanol Geotherm Res 25:181–191
Spycher N, Peiffer L, Sonnenthal E, Saldi G, Reed MH, Kennedy BM (2014) Integrated multicomponent solute geothermometry. Geothermics 51:113–123
Tonani F (1980) Some remarks on the application of geochemical techniques in geothermal exploration. In: Proceedings Adv. Eur. Geoth. Res., Second symp., Strasbourg, pp 428–443
Verma SP, Pandarinath K, Santoyo E (2008) SolGeo: a new computer program for solute geothermometers and its application to Mexican geothermal fields. Geothermics 37(6):597–621
Vespasiano G, Notaro P, Cianflone G (2018) Water-mortar interaction in a tunnel located in the Southern Calabria (Southern Italy). Environ Eng Geosci 24(3):305–315
Xu T, Hou Z, Jia X, Spycher N, Jiang Z, Feng B, Na J, Yuan Y (2016) Classical and integrated multicomponent geothermometry at the Tengchong geothermal field, Southwestern China. Environ Earth Sci 75:1502. https://doi.org/10.1007/s12665-016-6298-6
Acknowledgements
The authors (SC, AJ and UKS) wish to acknowledge Dr. P.K. Pujari, AD, RC&IG, for his support and contribution during the study. The tritium measurements carried out by Shri H.V. Mohokar and Smt. Diksha Pant are also gratefully acknowledged. The authors would like to thank all the officers of GSI associated with this project for their active cooperation in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chatterjee, S., Sinha, U.K., Biswal, B.P. et al. Multicomponent Versus Classical Geothermometry: Applicability of Both Geothermometers in a Medium-Enthalpy Geothermal System in India. Aquat Geochem 25, 91–108 (2019). https://doi.org/10.1007/s10498-019-09355-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-019-09355-w