Abstract
Purpose
To investigate brain morphological alterations of high-tension glaucoma patients and explore the association between brain morphological changes and elevated intraocular pressure.
Methods
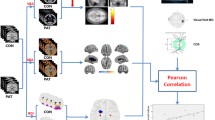
Thirty-six patients with high-tension glaucoma and 20 healthy controls were collected and underwent structural MRI scan. Surface-based morphometry and voxel-based morphometry were applied to assess cortical thickness and subcortical gray matter volume of the enrolled subjects. The association between brain morphometry and intraocular pressure was assessed by partial correlation.
Results
Compared with healthy controls, high-tension glaucoma patients showed decreased cortical thickness in the bilateral superior temporal gyrus, bilateral superior parietal gyrus, bilateral lateral occipital gyrus, left fusiform gyrus, left medial orbitofrontal gyrus, right precentral gyrus, and right superior frontal gyrus (p < 0.05). High-tension glaucoma patients also showed reduced gray matter volume in the right hippocampus, bilateral putamen, and bilateral thalamus (p < 0.05). In addition, brain morphological correlates of mean intraocular pressure were found in the left rostral middle frontal gyrus, right precentral gyrus, and left postcentral gyrus in high-tension glaucoma group (p < 0.05).
Conclusion
High-tension glaucoma patients experienced morphological reduction in the visual and nonvisual areas throughout the entire brain. Elevated intraocular pressure may contribute to the reduction of cortical thickness in certain areas in the progression of the disease.


Similar content being viewed by others
References
Joung JY, Lee WJ, Lee BR (2019) Comparison of blue and Green confocal scanning laser ophthalmoscope imaging to detect retinal nerve Fiber layer defects. Korean J Ophthalmol 33:131–137
Quigley HA (2011) Glaucoma. Lancet 377:1367–1377
Hindle AG, Thoonen R, Jasien JV, Grange RMH, Amin K, Wise J, Ozaki M, Ritch R, Malhotra R, Buys ES (2019) Identification of candidate miRNA biomarkers for Glaucoma. Invest Ophthalmol Vis Sci 60:134–146
Weinreb RN, Aung T, Medeiros FA (2014) The pathophysiology and treatment of glaucoma: a review. JAMA 311:1901–1911
Cheng J, Kong X, Xiao M, Sun X (2016) Twenty-four-hour pattern of intra-ocular pressure in untreated patients with primary open-angle glaucoma. Acta Ophthalmol 94:e460–e467
Mabuchi F, Sakurada Y, Kashiwagi K et al (2012) Association between genetic variants associated with vertical cup-to-disc ratio and phenotypic features of primary open-angle glaucoma. Ophthalmology 119:1819–1825
Song Y, Mu K, Wang J, Lin F, Chen Z, Yan X, Hao Y, Zhu W, Zhang H (2014) Altered spontaneous brain activity in primary open angle glaucoma: a resting-state functional magnetic resonance imaging study. PLoS One 9:e89493
Wang J, Li T, Sabel BA, Chen Z, Wen H, Li J, Xie X, Yang D, Chen W, Wang N, Xian J, He H (2016) Structural brain alterations in primary open angle glaucoma: a 3T MRI study. Sci Rep 6:18969
Nivison MP, Ericson NG, Green VM, Bielas JH, Campbell JS, Horner PJ (2017) Age-related accumulation of phosphorylated mitofusin 2 protein in retinal ganglion cells correlates with glaucoma progression. Exp Neurol 296:49–61
Murphy MC, Conner IP, Teng CY et al (2016) Retinal structures and visual cortex activity are impaired prior to clinical vision loss in glaucoma. Sci Rep 6:31464
Boucard CC, Hernowo AT, Maguire RP, Jansonius NM, Roerdink JB, Hooymans JM, Cornelissen FW (2009) Changes in cortical grey matter density associated with long-standing retinal visual field defects. Brain 132:1898–1906
Zhou P, Wang J, Li T, Wang N, Xian J, He H (2016) Abnormal interhemispheric resting-state functional connectivity in primary open-angle glaucoma. Conf Proc IEEE Eng Med Biol Soc 2016:4055–4058
Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R, CIGTS Study Group (2011) Intraocular pressure control and long-term visual field loss in the collaborative initial Glaucoma treatment study. Ophthalmology 118:1766–1773
Nuzzi R, Dallorto L, Rolle T (2018) Changes of visual pathway and brain connectivity in Glaucoma: a systematic review. Front Neurosci 12:363
Fukuda M, Omodaka K, Tatewaki Y et al (2018) Quantitative MRI evaluation of glaucomatous changes in the visual pathway. PLoS One 13:e0197027
Yu L, Xie B, Yin X et al (2013) Reduced cortical thickness in primary open-angle glaucoma and its relationship to the retinal nerve fiber layer thickness. PLoS One 8:e73208
Jiang MM, Zhou Q, Liu XY, Shi CZ, Chen J, Huang XH (2017) Structural and functional brain changes in early- and mid-stage primary open-angle glaucoma using voxel-based morphometry and functional magnetic resonance imaging. Medicine 96:e6139
Yotter RA, Dahnke R, Gaser C (2011) Topological correction of brain surface meshes using spherical harmonics. Hum Brain Mapp 32:1109–1124
Frezzotti P, Giorgio A, Motolese I et al (2014) Structural and functional brain changes beyond visual system in patients with advanced glaucoma. PLoS One 9:e105931
Dai H, Morelli JN, Ai F et al (2014) Resting-state functional MRI: functional connectivity analysis of the visual cortex in primary open-angle glaucoma patients. Hum Brain Mapp 34:2455–2463
Giorgio A, Zhang J, Costantino F, de Stefano N, Frezzotti P (2018) Diffuse brain damage in normal tension glaucoma. Hum Brain Mapp 39:532–541
Bogorodzki P, Piątkowskajanko E, Szaflik J et al (2014) Mapping cortical thickness of the patients with unilateral end-stage open angle glaucoma on planar cerebral cortex maps. PLoS One 9:e93682
Murray EA, Mishkin M (1984) Severe tactual as well as visual memory deficits follow combined removal of the amygdala and hippocampus in monkeys. Brain Res 270:340–344
Li C, Cai P, Shi L, Lin Y, Zhang J, Liu S, Xie B, Shi Y, Yang H, Li S, du H, Wang J (2012) Voxel-based morphometry of the visual-related cortex in primary open angle glaucoma. Curr Eye Res 37:794–802
Steinmetz MA, Constantinidis C (1995) Neurophysiological evidence for a role of posterior parietal cortex in redirecting visual attention. Cereb Cortex 5:448–456
Chen WW, Wang N, Cai S et al (2013) Structural brain abnormalities in patients with primary open-angle glaucoma: a study with 3T MR imaging. Invest Ophthalmol Vis Sci 54:545–554
Chen Z, Lin F, Wang J et al (2013) Diffusion tensor magnetic resonance imaging reveals visual pathway damage that correlates with clinical severity in glaucoma. Aust NZ J Ophthalmol 41:43–49
Du BF, Levy R, Volle E et al (2006) Functions of the left superior frontal gyrus in humans: a lesion study. Brain 129:3315–3328
Johnson PB, Ferraina S, Bianchi L et al (1996) Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex 6:102–109
Paus T (1996) Location and function of the human frontal eye-field: a selective review. Neuropsychologia 34:475–483
Burgess N, Maguire EA, O'Keefe J (2002) The human hippocampus and spatial and episodic memory. Neuron 35:625–641
Zikou AK, Kitsos G, Tzarouchi LC, Astrakas L, Alexiou GA, Argyropoulou MI (2012) Voxel-based morphometry and diffusion tensor imaging of the optic pathway in primary open-angle glaucoma: a preliminary study. Am J Neuroradiol 33:128–134
DeJong LW, Van der Hiele K, Veer IM et al (2008) Strongly reduced volumes of putamen and thalamus in Alzheimer's disease: an MRI study. Brain 131:3277–3285
Wang WH, Mcnatt LG, Pang IH et al (2008) Increased expression of serum amyloid a in glaucoma and its effect on intraocular pressure. Invest Ophthalmol Vis Sci 49:1916–1923
Gupta N, Ang LC, Tilly LND et al (2006) Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol 90:674
Michalski LJ, Demers CH, Baranger DAA et al (2017) Perceived stress is associated with increased rostral middle frontal gyrus cortical thickness: a family-based and discordant-sibling investigation. Genes Brain Behav 16:781–789
Whelan CD, Altmann A, Botía JA, Jahanshad N, Hibar DP, Absil J, Alhusaini S, Alvim MKM, Auvinen P, Bartolini E, Bergo FPG, Bernardes T, Blackmon K, Braga B, Caligiuri ME, Calvo A, Carr SJ, Chen J, Chen S, Cherubini A, David P, Domin M, Foley S, França W, Haaker G, Isaev D, Keller SS, Kotikalapudi R, Kowalczyk MA, Kuzniecky R, Langner S, Lenge M, Leyden KM, Liu M, Loi RQ, Martin P, Mascalchi M, Morita ME, Pariente JC, Rodríguez-Cruces R, Rummel C, Saavalainen T, Semmelroch MK, Severino M, Thomas RH, Tondelli M, Tortora D, Vaudano AE, Vivash L, von Podewils F, Wagner J, Weber B, Yao Y, Yasuda CL, Zhang G, Bargalló N, Bender B, Bernasconi N, Bernasconi A, Bernhardt BC, Blümcke I, Carlson C, Cavalleri GL, Cendes F, Concha L, Delanty N, Depondt C, Devinsky O, Doherty CP, Focke NK, Gambardella A, Guerrini R, Hamandi K, Jackson GD, Kälviäinen R, Kochunov P, Kwan P, Labate A, McDonald C, Meletti S, O'Brien TJ, Ourselin S, Richardson MP, Striano P, Thesen T, Wiest R, Zhang J, Vezzani A, Ryten M, Thompson PM, Sisodiya SM (2018) Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain 141:391–408
Morris VL, Owens MM, Syan SK et al (2019) Associations between drinking and cortical thickness in younger adult drinkers: findings from the human Connectome Project. Alcohol Clin Exp Res
Papmeyer M, Giles S, Jessica E, Sussmann et al (2015) Cortical thickness in individuals at high familial risk of mood disorders as they develop major depressive disorder. Biol Psychiatry 78:58–66
Zhu F, Tang L, Zhu P, Lin Q, Yuan Q, Shi W, Li B, Ye L, Min Y, Su T, Shao Y (2019) Resting-state functional magnetic resonance imaging (fMRI) and functional connectivity density mapping in patients with corneal ulcer. Neuropsychiatr Dis Treat 15:1833–1844
Grosbras MH, Laird AR, Paus T (2005) Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Mapp 25:140–154
Funding
This study was funded by the Key Research and Development Program of Shandong Province (Grant No. 2017GGX201010), the Natural Science Foundation of Shandong Province (Grant No. ZR2016HM73) and the Academic Promotion Programme of Shandong First Medical University (Grant No. 2019QL009). JQ was supported by the Taishan Scholars Program of Shandong Province (Grant No. TS201712065).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, X., Zhou, J. et al. Brain morphological alterations of cerebral cortex and subcortical nuclei in high-tension glaucoma brain and its associations with intraocular pressure. Neuroradiology 62, 495–502 (2020). https://doi.org/10.1007/s00234-019-02347-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02347-1