Abstract
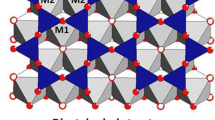
The versatile structure of smectites can exhibit large variations in chemical compositions and cationic substitutions in different crystallographic sites, resulting in various locations of layer charge. Natural smectites can contain various amounts of structural iron, the chemical form of which can influence the reactivity of these minerals. The variety of Fe crystal chemistry in smectite was explored for eight natural smectites of distinct chemical compositions and charge locations, together with two synthetic ferric saponites used as reference compounds for tetrahedral Fe(III). All samples were identified as dioctahedral or trioctahedral smectite by X-ray diffraction and Fourier-transform infrared spectroscopy. The extent of [4]Al for [4]Si substitution was determined by 27Al and 29Si magic angle spinning nuclear magnetic resonance spectroscopy. The Fe local chemical environment was probed by polarized X-ray absorption spectroscopy. Only Fe(III) could be detected in all samples, with no evidence of cluster formation. The O shell at 1.86 Å in synthetic saponites suggests Fe insertion in tetrahedral sites, and the absence of detected octahedral Fe implies quantitative substitution of [4]Fe3+ for [4]Si4+. In natural smectites, Fe(III) is bound to six O atoms at ~ 2.00 Å, suggesting insertion in octahedral sites. This inference is also supported by the detection of in-plane Mg/Al/Fe atoms at ~ 3.05 Å and out-of-plane Si/Al atoms at ~ 3.25 Å. In one Fe-rich nontronite, the detection of an O subshell at ~ 1.88 Å suggests a concomitant insertion of Fe(III) in tetrahedral sites. Low numbers of octahedral neighbors were detected in natural saponite and hectorite, presumably because of the presence of vacancies and/or Li(I) in adjacent octahedral sites balancing the local charge excess originating from the substitution of Fe(III) for Mg(II). The substitution of [4]Fe3+ for [4]Si4+ can be readily obtained under defined conditions in the laboratory, but seems more rare in natural samples, or present in amounts below the detection limit of spectroscopic methods used in this study.













Similar content being viewed by others
References
Ankudinov AL, Ravel B, Rehr JJ, Conradson SD (1998) Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys Rev B 58(12):7565–7576
Bailey SW (1980) Structures of layer silicates. In: Brindley GW, Brown G (eds) Crystal structures of clay minerals and their X-Ray identification. Mineralogical society, London, pp 2–123
Baron F, Petit S, Tertre E, Decarreau A (2016) Infuence of aqueous Si and Fe speciation on tetrahedral Fe(III) substitutions in nontronites: a clay synthesis approach. Clays Clay Miner 64(3):230–244
Bishop ME, Dong HL, Kukkadapu RK, Liu CX, Edelmann RE (2011) Bioreduction of Fe-bearing clay minerals and their reactivity toward pertechnetate (Tc-99). Geochim Cosmochim Acta 75(18):5229–5246
Breu J, Seidl W, Stoll A (2003) Disorder in smectites in dependence of the interlayer cation. Z Anorg Allg Chem 629(3):503–515
Brindley GW (1980) Order-disorder in clay mineral structures. In: Brindley GW, Brown G (eds) Crystal structures of clay minerals and their X-ray identification. Mineralogical Society, London, pp 125–195
Cadars S, Guégan R, Garaga MN, Bourrat X, Le Forestier L, Fayon F, Huynh TV, Allier T, Nour Z, Massiot D (2012) New insights into the molecular structures, compositions, and cation distributions in synthetic and natural montmorillonite clays. Chem Mater 24(22):4376–4389
Dong HL, Jaisi DP, Kim J, Zhang GX (2009) Microbe-clay mineral interactions. Am Miner 94(11–12):1505–1519
Drits VA, Manceau A (2000) A model for the mechanism of Fe3+ to Fe2+ reduction in dioctahedral smectites. Clays Clay Miner 48(2):185–195
Ernstsen V, Gates WP, Stucki JW (1998) Microbial reduction of structural iron in clays—a renewable source of reduction capacity. J Environ Qual 27(4):761–766
Favre F, Tessier D, Abdelmoula M, Génin JM, Gates WP, Boivin P (2002) Iron reduction and changes in cation exchange capacity in intermittently waterlogged soil. Eur J Soil Sci 53(2):175–183
Fialips CI, Huo D, Yan LB, Wu J, Stucki JW (2002) Infrared study of reduced and reduced-reoxidized ferruginous smectite. Clays Clay Miner 50(4):455–469
Finck N, Schlegel ML, Bosbach D (2009) Sites of Lu(III) sorbed to and coprecipitated with hectorite. Environ Sci Technol 43(23):8807–8812
Finck N, Schlegel ML, Bauer A (2015) Structural iron in dioctahedral and trioctahedral smectites: a polarized XAS study. Phys Chem Miner 42(10):847–859
Gates WP (2005) Infrared spectroscopy and the chemistry of dioctahedral smectites. In: Kloprogge T (ed) Vibrational spectroscopy of layer silicates and hydroxides, CMS workshop lecture series, vol 13. The Clay Mineral Society, Chantilly, pp 125–168
Gates WP (2008) Cation mass-valence sum (CM-VS) approach to assigning OH-bending bands in dioctahedral smectites. Clays Clay Miner 56(1):10–22
Gates WP, Stucki JW, Kirkpatrick RJ (1996) Structural properties of reduced Upton montmorillonite. Phys Chem Miner 23(8):535–541
Gates WP, Slade PG, Manceau A, Lanson B (2002) Site occupancies by iron in nontronites. Clays Clay Miner 50(2):223–239
Gorski CA, Klupfel LE, Voegelin A, Sander M, Hofstetter TB (2013) Redox properties of structural Fe in clay minerals: 3. Relationships between smectite redox and structural properties. Environ Sci Technol 47(23):13477–13485
Hofstetter TB, Neumann A, Schwarzenbach RP (2006) Reduction of nitroaromatic compounds by Fe(II) species associated with iron-rich smectites. Environ Sci Technol 40(1):235–242
Jaisi DP, Dong H, Plymale AE, Fredrickson JK, Zachara JM, Heald S, Liu C (2009) Reduction and long-term immobilization of technetium by Fe(II) associated with clay mineral nontronite. Chem Geol 264(1):127–138
Joe-Wong C, Brown GE, Maher K (2017) Kinetics and products of chromium(VI) reduction by iron(II/III)-bearing clay minerals. Environ Sci Technol 51(17):9817–9825
Kaufhold S, Stucki JW, Finck N, Steininger R, Zimina A, Dohrmann R, Ufer K, Pentrák M, Pentráková L (2017) Tetrahedral charge and Fe content in dioctahedral smectites. Clay Miner 52(1):51–65
Keeling JL, Raven MD, Gates WP (2000) Geology and characterization of two hydrothermal nontronites from weathered metamorphic rocks at the Uley Graphite Mine, South Australia. Clays Clay Miner 48(5):537–548
Khaled EM, Stucki JW (1991) Iron oxidation state effects on cation fixation in smectites. Soil Sci Soc Am J 55(2):550–554
Komarneni S, Fyfe CA, Kennedy GJ, Strobl H (1986) Characterization of synthetic and naturally-occuring clays by Al-27 and Si-29 magic angle spinning NMR spectroscopy. J Am Ceram Soc 69(3):C45–C47
Labouriau A, Kim YW, Earl WL (1996) Nuclear-spin-lattice relaxation in natural clays via paramagnetic centers. Phys Rev B 54(14):9952–9959
Lear PR, Stucki JW (1989) Effects of iron oxidation state on the specific surface area of nontronite. Clays Clay Miner 37(6):547–552
Lippmaa E, Mägi M, Samson A, Engelhardt G, Grimmer A-R (1980) Structural studies of silicates by solid-state high-resolution 29Si NMR. J Am Chem Soc 102(15):4889–4893
Madejova J, Komadel P (2001) Baseline studies of the clay minerals society source clays: infrared methods. Clays Clay Miner 49(5):410–432
Manceau A (1990) Distribution of cations among the octahedra of phyllosilicates—insight from EXAFS. Can Mineral 28:321–328
Manceau A, Schlegel ML (2001) Texture effect on polarized EXAFS amplitude. Phys Chem Miner 28(1):52–56
Manceau A, Chateigner D, Gates WP (1998) Polarized EXAFS, distance-valence least-squares modeling (DVLS), and quantitative texture analysis approaches to the structural refinement of Garfield nontronite. Phys Chem Miner 25(5):347–365
Manceau A, Lanson B, Drits VA, Chateigner A, Gates WP, Wu. J, Huo D, Stucki. JW (2000) Oxidation-reduction mechanism of iron in dioctahedral smectites: I. Crystal chemistry of oxidized reference nontronites. Am Miner 85(1):133–152
Mering J, Oberlin A (1967) Electron-optical study of smectites. Clays Clay Miner 15(1):3–25
Méring J, Glaeser R (1953) Sur le rôle de la valence des cations échangeables dans la montmorillonite. Bull Soc Fr Minéral Cristallogr 77:519–530
Mermut AR, Cano AF (2001) Baseline study of the clay minerals society source clays: chemical analyses of major elements. Clays Clay Miner 49(5):381–386
Meunier A (2005) Clays. Springer-Verlag, Berlin Heidelberg, p 472
Michot LJ, Bihannic I, Pelletier M, Rinnert E, Robert JL (2005) Hydration and swelling of synthetic Na-saponites: Influence of layer charge. Am Miner 90(1):166–172
Moore DM, Reynolds RC Jr (1997) X-ray diffraction and the identification and analysis of clay minerals. Oxford University Press, Oxford, p 400
Morris HD, Bank S, Ellis PD (1990) Al-27 NMR spectroscopy of iron-bearing montmorillonite clays. J Phys Chem 94(7):3121–3129
Munoz M, Vidal O, Marcaillou C, Pascarelli S, Mathon O, Farges F (2013) Iron oxidation state in phyllosilicate single crystals using Fe-K pre-edge and XANES spectroscopy: effects of the linear polarization of the synchrotron X-ray beam. Am Miner 98(7):1187–1197
Neumann A, Petit S, Hofstetter TB (2011) Evaluation of redox-active iron sites in smectites using middle and near infrared spectroscopy. Geochim Cosmochim Acta 75(9):2336–2355
Prietzel J, Thieme J, Eusterhues K, Eichert D (2007) Iron speciation in soils and soil aggregates by synchrotron-based X-ray microspectroscopy (XANES, mu-XANES). Eur J Soil Sci 58(5):1027–1041
Proux O, Nassif V, Prat A, Ulrich O, Lahera E, Biquard X, Menthonnex J-J, Hazemann J-L (2006) Feedback system of a liquid-nitrogen-cooled double-crystal monochromator: design and performances. J Synchrotron Radiat 13:59–68
Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12:537–541
Rothe J, Butirin S, Dardenne K, Denecke MA, Kienzler B, Löble M, Metz V, Seibert A, Steppert M, Vitova T, Walther C, Geckeis H (2012) The INE-beamline for actinide science at ANKA. Rev Sci Instrum 83(4):13
Sanz J, Serratosa JM (1984a) Distinction of tetrahedrally and octahedrally coordinated Al in phyllosilicates by NMR spectroscopy. Clay Miner 19(1):113–115
Sanz J, Serratosa JM (1984b) Si-29 and Al-27 high-resolution MAS NMR spectra of phyllosilicates. J Am Chem Soc 106(17):4790–4793
Schlegel ML, Manceau A (2013) Binding mechanism of Cu(II) at the clay-water interface by powder and polarized EXAFS spectroscopy. Geochim Cosmochim Acta 113:113–124
Schlegel ML, Manceau A, Chateigner D, Charlet L (1999) Sorption of metal ions on clay minerals I. Polarized EXAFS evidence for the adsorption of Co on the edges of hectorite particles. J Colloid Interface Sci 215(1):140–158
Semenova TF, Rozhdestvenskaya IV, Frankkamenetsky VA (1977) Refinement of the crystal structure of tetraferriphlogopite. Kristallografiya 22(6):1196–1201
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A 32(SEP1):751–767
Stucki JW, Roth CB (1977) Oxidation-reduction mechanism for structural iron in nontronite. Soil Sci Soc Am J 41(4):808–814
Stucki JW, Golden DC, Roth CB (1984a) Effects of reduction and reoxidation of structural iron on the surface area and dissolution of dioctahedral smectites. Clays Clay Miner 32(5):350–356
Stucki JW, Low PF, Roth CB, Golden DC (1984b) Effects of oxidation state of octahedral iron on clay swelling. Clays Clay Miner 32(5):357–362
Thomas J, Glass HD, White WA, Trandel RM (1977) Fluoride content of clay minerals and argillaceous earth materials. Clays Clay Miner 25(4):278–284
Tsipursky SI, Drits VA (1984) The distribution of octahedral cations in the 2:1 layers of dioctahedral smectites studied by oblique-texture elecron diffraction. Clay Miner 19(2):177–193
Vantelon D, Montarges-Pelletier E, Michot LJ, Briois V, Pelletier M, Thomas F (2003) Iron distribution in the octahedral sheet of dioctahedral smectites. An Fe K-edge X-ray absorption spectroscopy study. Phys Chem Miner 30(1):44–53
Waychunas GA, Apted MJ, Brown GE (1983) X-ray K-edge absorption spectra of Fe minerals and model compounds: Near-edge structure. Phys Chem Miner 10(1):1–9
Westre TE, Kennepohl P, DeWitt JG, Hedman B, Hodgson KO, Solomon EI (1997) A multiplet analysis of Fe K-edge 1 s→3d pre-edge features of iron complexes. J Am Chem Soc 119(27):6297–6314
Wilke M, Farges F, Petit PE, Brown GE, Martin F (2001) Oxidation state and coordination of Fe in minerals: an Fe K-XANES spectroscopic study. Am Miner 86(5–6):714–730
Woessner DE (1989) Characterization of clay minerals by Al-27 nuclear magnetic resonance spectroscopy. Am Miner 74(1–2):203–215
Acknowledgements
We acknowledge the contribution of the late Dr. J.-L. Robert of IMPMC to this work. We thank E. Soballa (KIT-INE) for SEM-EDX analyses. We acknowledge the KIT Synchrotron Light Source and the Institute for Beam Physics and Technology (IBPT) for operation of the storage ring, the Karlsruhe Research Accelerator (KARA). We also thank the ESRF for provision of synchrotron radiation beam time and I. Kieffer for support at the BM30B (ESRF) beamline.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Finck, N., Schlegel, M.L., Dardenne, K. et al. Structural iron in smectites with different charge locations. Phys Chem Minerals 46, 639–661 (2019). https://doi.org/10.1007/s00269-019-01028-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-019-01028-y