Abstract
Background
Lowe syndrome and Dent-2 disease are caused by mutations in the OCRL gene, which encodes for an inositol 5-phosphatase. The renal phenotype associated with OCRL mutations typically comprises a selective proximal tubulopathy, which can manifest as Fanconi syndrome in the most extreme cases.
Methods
Here, we report a 12-year-old male with nephrotic-range proteinuria and focal segmental glomerulosclerosis on renal biopsy. As a glomerular pathology was suspected, extensive investigation of tubular function was not performed.
Results
Surprisingly, whole exome sequencing identified a genetic variant in OCRL (c1467-2A>G) that introduced a novel splice mutation leading to skipping of exon 15. In situ hybridisation of adult human kidney tissue and zebrafish larvae showed OCRL expression in the glomerulus, supporting a role for OCRL in glomerular function. In cultured podocytes, we found that OCRL associated with the linker protein IPIP27A and CD2AP, a protein that is important for maintenance of the podocyte slit diaphragm.
Conclusion
Taken together, this work suggests a previously under-appreciated role for OCRL in glomerular function and highlights the importance of investigating tubular function in patients with persistent proteinuria.
Similar content being viewed by others
Introduction
The oculocerebrorenal syndrome of Lowe, also known as Lowe syndrome, is a rare X-linked disorder caused by mutations in the OCRL gene, which encodes for a type II inositol polyphosphate 5-phosphatase [1]. With respect to mortality, the most important phenotype in patients with Lowe syndrome is kidney dysfunction, which is characterised by a gradual decline in excretory renal function leading to chronic kidney disease (CKD) [2, 3]. The renal tubular phenotype can also include hypercalciuria, bicarbonate loss with consequent acidosis [2, 4] and presents as a spectrum from selective proximal tubulopathy to overt Fanconi syndrome [5]. Affected individuals also develop ocular defects (congenital cataracts) and central nervous system involvement [6, 7], as well as other features including post-natal growth retardation, muscular hypotonia and arthropathy in later life [5, 8].
The renal phenotypes associated with Lowe syndrome are also observed in Dent disease, which is an X-linked proximal tubulopathy characterised by low molecular weight proteinuria, hypercalciuria, nephrocalcinosis and progressive CKD [3, 9, 10]. Other features of proximal tubular dysfunction, such as aminoaciduria, glycosuria and complete Fanconi syndrome, can occur in Dent disease but are less frequent compared to Lowe syndrome [5, 11]. There are two forms of Dent disease, with the most common caused by mutations in the renal chloride transporter CLCN5 (Dent-1 disease) and the less common type caused by mutations in OCRL (Dent-2 disease) [3, 12,13,14]. Dent-2 disease presents as a clinical intermediate between Lowe syndrome and Dent-1 disease [3, 12] and lacks the most overt clinical features of Lowe syndrome [15]. Mild or sub-clinical extra-renal manifestations have been reported with Dent-2 disease and include peripheral cataracts, intellectual disability and short stature [5, 8, 16]. This overlap of clinical phenotypes between Lowe syndrome and Dent-2 disease is indicative of the heterogeneity of OCRL mutations.
Proximal tubulopathy is considered to be the primary renal feature associated with Lowe syndrome and Dent-2 disease, yet atypical renal phenotypes including glomerular dysfunction have also been reported. Kaneko et al. describe a child presenting with persistent proteinuria whose renal biopsy histology led to a presumptive diagnosis of idiopathic focal segmental glomerulosclerosis (FSGS) [17]. However, low-molecular weight urinary proteins were persistently high and subsequent genetic investigations identified a novel OCRL mutation. In the absence of extra-renal manifestations characteristic of Lowe syndrome, a diagnosis of Dent-2 disease was made [17]. A mutation in CLCN5, which is expressed in podocytes [18], has also been identified in Dent-1 patients presenting with nephrotic range proteinuria and FSGS on renal biopsy [19,20,21], and in a recent review by van Berkel et al., glomerulosclerosis was detected in almost two thirds of patients with Dent disease [22]. Nephrotic-range proteinuria [22] has been reported in several other studies of novel and known pathogenic OCRL mutations [15, 16, 22, 23]. Taken together, these findings fit with the current consensus that glomerular dysfunction in Lowe syndrome and Dent disease is a reported feature but it is considered to be secondary to the tubulointerstitial disease [8, 22, 24,25,26].
In this study, we report the case of a patient who presented with nephrotic-range proteinuria and FSGS. Surprisingly, genetic analysis identified a splicing mutation in OCRL. Given the predominant glomerular pathology on renal biopsy, we explored a possible role for OCRL in glomerular function. We show that OCRL is expressed in podocytes in vivo and use cultured podocytes to understand better the role of OCRL in this cell type. Interestingly, we found in cultured podocytes that OCRL associates with CD2AP, a protein that has an important role in maintaining the glomerular slit diaphragm. Overall, these findings suggest OCRL affects podocyte function directly.
Methods
Ethical approval
For use of normal human biopsy sections (see detailed methods below), ethical approval was through the Manchester Renal Biobank reference: 16/NW/019.
Cell culture
Conditionally, immortalised human podocytes generated by Saleem et al. [27] were cultured at the permissive temperature of 33 °C in RPMI-1640 medium with glutamine (Sigma, MO, USA) supplemented with 10% FBS (v/v) and ITS. Upon reaching 70–80% confluence, proliferating cells were thermo-switched to 37 °C for 10–14 days to allow differentiation of cells. Mesangial cells [28] were cultured in the same medium as the podocyte cells at 37 °C for 5 days. Glomerular endothelial cells [29] were cultured in EBM-2 medium (Lonza, UK) supplemented with 5% (v/v) FBS and EBM2 bullet kit (Lonza, UK) growth factors, excluding VEGF, at 37 °C for 5 days.
Preparation of cell lysates and protein extraction
Cells were washed with ice-cold PBS and lysed with HMNT buffer plus protease inhibitors (20 mM HEPES, pH 7.4, 5 mM MgCl2, 0.1 M NaCl, 0.5% (w/v) Triton X-100). Cell extracts were centrifuged at 14,000×g at 4 °C for 10 min, and the supernatant was kept on ice until needed.
SDS-PAGE and Western blot analysis
Cell lysates from differentiated wild-type podocytes, glomerular endothelial cells and mesangial cells were analysed by SDS-PAGE on 4–12% Bis-Tris gels (Life Sciences). Proteins were transferred to nitrocellulose membranes, and endogenous OCRL was blotted using polyclonal anti-OCRL (1:500 dilution, Lowe lab [30]) followed by anti-sheep Alexa Fluor-conjugated secondary antibody (1:5000 dilution, Life Technologies Ltd). Immunoblotted proteins were detected using Odyssey infrared imaging system (LI-COR, Biosciences UK Ltd.).
Immunoprecipitation of endogenous OCRL and CD2AP
Protein-G Dynabeads (Life Technologies) were re-suspended, and the supernatant was discarded. Anti-OCRL (1:200 dilution, Santa Cruz Biotechnology, SC-393577) or anti-CD2AP (1:200 dilution, Santa Cruz Biotechnology, SC-9137) antibody, diluted in PBS with 0.02% Tween, was added to the beads and incubated by rotation at 4 °C for 1 h. Fresh total cell lysate (TCL) was added to the suspension, which was further incubated by rotation at 4 °C for 4 h. The beads were washed, and proteins were eluted from the beads with sample buffer (5×) at 95 °C for 10 min. Samples were separated by electrophoresis, and proteins were identified by immunoblotting with anti-OCRL or anti-CD2AP followed by appropriate species-specific Alexa Fluor-conjugated secondary antibody (1:5000 dilution, Life Technologies Ltd.).
Endogenous protein pull down
Glutathione S-transferase (GST)-tagged recombinant IPIP27A or GST alone (Lowe lab [31]) was coupled to glutathione-sepharose beads (GE Healthcare, UK). Following incubation with rotation at 4 °C for 1 h, protein-coupled beads were washed and centrifuged at 9000 rpm at 4 °C for 1 min and the supernatant was discarded. Fresh TCL was added to the protein-coupled beads and following incubation with rotation at 4 °C for 3 h, samples were centrifuged at 9000 rpm at 4 °C for 1 min. Proteins were eluted from the beads with sample buffer (5×) at 95 °C for 10 min. Samples were separated by electrophoresis, and proteins were identified by immunoblotting with anti-OCRL or anti-CD2AP (as described above), followed by anti-sheep Alexa Fluor-conjugated secondary antibody (1:5000 dilution, Life Technologies Ltd).
Zebrafish husbandry
Zebrafish were maintained and staged according to established protocols [32] and in accordance with the personal project license of Professor Martin Lowe (70/9091) and under the current guidelines of the UK Animals Act 1986. Embryos were collected from group-wise matings of wild-type AB Notts.
In situ hybridisation
Whole-mount in situ hybridisation on zebrafish embryos was performed as previously described (Thisse Thisse, Nat Protocols, 2008). Digoxigenin-labelled anti-sense riboprobes were made using T3 RNA polymerase transcription kits (Roche Diagnostics). Probe templates for zebrafish ocrl and human OCRL were generated by PCR amplification from cDNA taken from 5 dpf zebrafish embryos and human breast tissue, respectively. The primers used were as follows: F zfish ocrl 5′-CTCTGGAAACTACCTGCCCA-3′, R zfish ocrl 5′-GGATCCAATTAACCCTCACTAAAGGGCATTCGAGACAGCGCTGAAA-3′; F human OCRL 5′-CAGTGAGAGACCCCTTCAGG-3′, R human OCRL 5′-GGATCCAATTAACCCTCACTAAAGGGGGAACTGAATAGCACGCTGG-3′. Note that on the reverse primers, a T3 anchor sequence (GGATCCAATTAACCCTCACTAAAGGG) was used to enable T3-mediated RNA synthesis off the purified PCR product. For human kidney sections, the same protocol used in zebrafish was performed but slides with de-waxed kidney sections were prepared with a 30-min Proteinase K (3 μg/ml in PBS) incubation at 37 °C followed by two PBS washes and 15 min 0.2 M HCl incubation at RT°C. Slides were washed a further two times in PBS, and then, the in situ protocol was followed. Probe incubations were performed with 100 μl of RNA/Hyb+ underneath a coverslip.
Genetic studies
Samples were collected following informed consent. OCRL Sanger sequencing and analysis of X-inactivation were performed as previously described. For whole-exome sequencing, library preparation, sequencing and data generation were performed in the Genomics Core Facility of the Biomedical Research Centre at Guy’s and St. Thomas’ Hospitals and King’s College London. DNA libraries were prepared from 3 μg dsDNA using SureSelect Human All Exon 50 Mb Kit (Agilent Technologies). Samples were multiplexed (four samples on each lane), and 100 base pair paired end sequencing was performed on Illumina HiSeq system. Sequence data were aligned to the human reference genome using Novoalign, and variants were called with SAMtools and annotated via multiple passes through Annovar. Exome sequencing data analysis was performed at the University of Bristol (Academic Renal Unit) [33]. Variants of interest were confirmed using Sanger sequencing (Eurofins MWG Operon, Germany). The KAPA HiFi PCR Kit (Kapa Biosystems) was used for the amplification.
Results and discussion
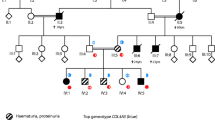
We report the index case of a 12-year-old male with persistent proteinuria, renal impairment and FSGS on renal biopsy. The patient presented with a high serum creatinine (99 μmol/l), normal serum albumin (49 g/l) but elevated urine albumin:creatinine ratio (ACR) (70–94 mg/mmol) and protein:creatinine ratios (PCR) (160–330 mg/mmol). The difference between the PCR and the ACR is suggestive of tubular proteinuria; however, this was not formally investigated and the patient proceeded to have a renal biopsy. This revealed 9 sclerosed glomeruli from 23, representing 39% sclerosis. Two more glomeruli showed adherence of the glomerular tuft to the Bowman’s capsule and other glomeruli showed signs of mesangial hypercellularity and increased mesangial matrix deposition. The tubules contained some proteinaceous material, and some had blood cells present within the lumen but tubular morphology was generally normal apart from slight atrophic changes in three tubule profiles. Three medium-sized arteries present within the biopsy were also normal, and the interstitium showed mild expansion without fibrosis or the presence of foam cells. Immunofluorescence on six glomeruli was negative for IgG, IgA, Fibrinogen, C1Q, C3, Kappa and Lambda. Electron microscopy revealed foot process effacement and irregular in-folding of the glomerular basement membrane. No tubular pathology was documented. Since the clinical and biopsy findings were suggestive of a glomerular disorder, tubular dysfunction was not thoroughly examined and so we cannot discount that further investigation may have confirmed tubular dysfunction. However, from the available results, there was no overt evidence of proximal tubular dysfunction; urinary pH, serum calcium and glucose levels were within normal ranges, as were serum bicarbonate and phosphate levels and an ultrasound scan of the urinary tract did not show nephrocalcinosis. The patient was treated with the angiotensin converting enzyme (ACE) inhibitor enalapril and has experienced several episodes of AKI, which responded to withdrawal of ACE inhibition.
Interestingly, an older male sibling presented to the adult service at a similar time to the index case but with severe acute kidney injury (AKI) and a creatinine of 1000 μmol/l. He had been given a presumptive diagnosis of IgA nephropathy at a younger age when the family lived in Poland, and he had been receiving treatment with the ACE inhibitor, ramipril. At presentation with severe AKI, his serum albumin was within the normal range, his urine protein:creatinine ratio was mildly elevated (60 mg/mmol) and he did not have further tests to investigate tubular function. His biopsy revealed 7 out of 7 normal glomeruli but widespread acute tubular necrosis. His renal ultrasound scan did not have evidence of nephrocalcinosis. He did not recover renal function and he subsequently received dialysis and transplantation.
Whole-exome sequencing of the index case revealed a variant of OCRL on the X chromosome (c1467-2A>G) [33]. This mutation is a hemizygous one base pair substitution located at the acceptor site of intron 14. Subsequently, this intronic splice mutant was confirmed in both the index case and the sibling by Sanger sequencing (Fig. 1a) and was also identified in the unaffected heterozygote mother. In silico analysis predicts that this mutation abolishes the acceptor site of intron 14 to induce skipping of exon 15 (Fig. 1b). From a panel of 53 known genes associated with steroid-resistant nephrotic syndrome, no other mutations were identified [33], implying that the OCRL variant was the cause of the renal pathology observed. The index case had short stature and was treated with growth hormone treatment. Short stature is a common feature of Lowe syndrome, but this patient lacked ocular and neurological abnormalities. Given the nephrotic-range proteinuria and the histological pattern of FSGS, we propose that this OCRL mutation represents a mild form of Lowe syndrome or Dent-2 disease.
Genetic analysis identifies a novel splice mutant in OCRL. a Chromatogram’s from Sanger sequencing showing the c1467-2A>G mutation (highlighted red) detected in the index patient, sibling and heterozygous mother. Top panel shows non-mutant sequence and the corresponding intron exon boundary. b Schematic representation of splicing outcomes for normal OCRL (top) and the index patient OCRL sequence containing the c1467-2A>G mutation (bottom). Use of the Spliceport predictive tool [34] showed a strong splice acceptor site on exon 15 in the normal OCRL (+ 1.57), which is completely lost in the variant OCRL (− 2.23) (for reference the splice acceptor values for exon 14 and exon 16 are also shown (black), these were unchanged in the variant). Three other splicing predictive tools (SpliceSiteFinder-like, MaxEntScan [35] and NNSPLICE [36], which are algorithms run on the bioinformatics interface Alamut) also showed the OCRL variant abolished the splice acceptor site (data not shown). Taken together, these programs strongly suggest that the mature transcript of the OCRL variant will undergo exon skipping of exon 15 to yield the mature transcript illustrated (right)
As the index patient presented with a predominant glomerular pathology on renal biopsy, we proceeded to investigate further a role for OCRL in the glomerulus. Previous studies have demonstrated the expression of OCRL to be ubiquitous, and transcripts have been identified in the kidney [37, 38]. We aimed to confirm previous findings and to better understand the spatial distribution of glomerular OCRL transcripts in vivo in two different vertebrates (human and zebrafish). To do this, we performed in situ hybridisation on 5 days post fertilisation zebrafish, which is an embryonic stage when the glomerulus has fully formed [39]. ocrl expression in the zebrafish embryo was detected at high levels in the gut, eyes, swim bladder, neural tube, ventral fin mesenchyme, branchial arches and the forebrain, midbrain and hindbrain (Fig. 2a). We also found ocrl transcripts in the zebrafish glomerulus when analysed in transverse section (Fig. 2a). These data suggest OCRL functions in the glomerulus from the earliest stages of development. We also performed in situ hybridisation on histological sections of normal human renal biopsy tissue. We detected OCRL transcripts in all cell types within the glomerulus (mesangial cells, endothelial cells and podocytes; Fig. 2b). In summary, we confirm the presence of OCRL transcripts in glomerular cells in vivo and our analysis in zebrafish suggests that OCRL functions from early stages of development in vertebrates.
In situ hybridisation detects OCRL expression in the glomeruli of zebrafish and adult human kidney tissue. a Top panels show OCRL expression in a whole-mount zebrafish embryo (left panel, lateral view, anterior to the left) and in transverse section through the glomerulus (right panel, highlighted with white dotted outline) at 5 days post fertilisation (dpf). b In situ hybridisation of normal adult human kidney tissue showing OCRL expression within the glomerulus. The left panel shows a low magnification image with glomeruli and proximal tubules labelled. The right panel shows a close-up view of the glomerulus with OCRL expression labelled in podocytes (black arrow), mesangial cells (green arrow) and endothelial cells (red arrow)
In order to investigate the biochemical interactions of OCRL in glomerular cells, we adopted an in vitro approach. Using Western blot analyses, we found OCRL protein in cultured endothelial cells, mesangial cells and podocytes (Fig. 3a). To elucidate the protein-protein interactions of OCRL in cultured podocytes, we performed immunoprecipitation and protein pull-down experiments on these cells to isolate interacting partners of OCRL. We found that OCRL co-immunoprecipitates with the slit diaphragm protein CD2AP in cultured podocytes (Fig. 3 b and c). Interaction between these proteins is likely to be mediated by the linker protein IPIP27A, which can bind to both OCRL and CD2AP in pull-down experiments (Fig. 3 d and e), in agreement with findings in other cell types [31, 40, 41]. CD2AP is a component of the glomerular filtration apparatus in the kidney, localising to nephrin and podocin protein bridges to maintain the slit diaphragm between adjacent podocyte foot processes [42,43,44]. Mutations in CD2AP cause glomerular diseases associated with defective trafficking within the endocytic pathway [45]. We predict that the interaction between OCRL and CD2AP is important in the regulation of endocytic trafficking, actin cytoskeleton dynamics and maintenance of the slit diaphragm. In support of this, endocytic processes in the podocyte play a fundamental role in the development and maintenance of the glomerular filtration barrier [46, 47]. Alternatively, the OCRL-IPIP27A-CD2AP protein complex may function directly at the unique podocyte cell-cell junctions in order to maintain the slit diaphragm. Given that CD2AP is crucial in maintaining the integrity of the glomerular filter [48, 49], disruption of this protein complex in Lowe syndrome or Dent-2 disease may directly lead to podocyte injury and glomerular proteinuria. In our patient case, the pathology was caused by an exon 15 skipping mutation. This is the commonest exon for reported OCRL mutations but is not in the domain where IPIP27A binds (which is exons 21–22).
Immunoprecipitation and protein pull-down analysis showing interaction between CD2AP, OCRL and IPIP27A. a Western blot analysis showing that OCRL is abundant in cultured glomerular cells (endothelial cells, mesangial cells and podocytes). b, c Co-immunoprecipitation experiments using control antibody (IgG) or antibodies to OCRL (b) or CD2AP (c) demonstrating interaction between the endogenous proteins. d, e Protein pull-downs using GST control or GST-IPIP27A as bait showing binding to endogenous OCRL (d) and CD2AP (e). Glutathione S-transferase (GST), immunoprecipitation (IP)
The glomerular phenotype observed in the index case may therefore be a consequence of the heterogeneity of OCRL mutations. Our patient study therefore adds to the reports that already exist that show the same mutation in OCRL does not correlate with disease severity [23]. Another possibility is that exon 15 skipping causes protein instability that diminishes the IPIP27A binding, thus resulting in a glomerular phenotype due to CD2AP mislocalisation or function. OCRL is also involved in actin dynamics and endosomal trafficking through is 5-phosphatase activity that converts PtdIns(4,5)P2 to PtdIns4P. As slit diaphragm formation requires endosomal trafficking and actin polymerisation, we cannot rule out the possibility that non-IPIP27A interactions by OCRL are also required for maintenance of podocyte function.
Our results raise the possibility that defective OCRL can directly cause a glomerulopathy. In support of this, we show that OCRL is expressed in podocytes in vivo and is able to interact with CD2AP, an important protein whose function is required for intact glomerular function. The potential of glomerular dysfunction in patients with Lowe syndrome or Dent-2 disease hints that caution should be given to how patients are treated as further study is required to confirm that glomerular phenotypes are directly caused by perturbed OCRL function in podocytes. We suggest that treatment of disease-causing OCRL mutations could be stratified based on biochemical and genetic analyses. Confirmation of an OCRL mutation in the presence of nephrotic-range proteinuria could be used initially to determine the therapeutic approach. For example, inhibition of the renin-angiotensin system would not significantly benefit low molecular weight proteinuria (indicative of tubular dysfunction), yet there are reports of improvement in proteinuria after commencing enalapril therapy in a patient with Dent disease [21], supporting a therapeutic approach targeting a glomerular origin for proteinuria.
We also propose, in agreement with Copelovitch et al. [21], that OCRL and CLCN5 be added to the list of genes tested prior to renal biopsy in young males presenting with proteinuria (including those with nephrotic-range), who do not have hypoalbuminemia or oedema and once tubular proteinuria has been evaluated. Moreover, nephrotic range proteinuria is associated with other tubular disease genes, such as CUBN [50], and these genes should similarly be added to the gene list in order to better guide the physician in their diagnoses and treatment options. This genetic information will be beneficial in avoiding the need for renal biopsy and in preventing exposure to immunosuppressive therapies and their undesirable side effects. The genetic diagnoses and appropriate management of tubular versus glomerular proteinuria may also delay end-stage renal disease in these patients. However, this requires a thorough evaluation of both tubular and glomerular function in patients who present with persistent proteinuria.
References
Attree O, Olivos IM, Okabe I, Bailey LC, Nelson DL, Lewis RA, McInnes RR, Nussbaum RL (1992) The Lowe’s oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 358:239–242. https://doi.org/10.1038/358239a0
Bockenhauer D, Bokenkamp A, van’t Hoff W, Levtchenko E, Kist-van Holthe JE, Tasic V, Ludwig M (2008) Renal phenotype in Lowe syndrome: a selective proximal tubular dysfunction. Clin J Am Soc Nephrol 3:1430–1436. https://doi.org/10.2215/cjn.00520108
Bokenkamp A, Bockenhauer D, Cheong HI, Hoppe B, Tasic V, Unwin R, Ludwig M (2009) Dent-2 disease: a mild variant of Lowe syndrome. J Pediatr 155:94–99. https://doi.org/10.1016/j.jpeds.2009.01.049
Charnas LR, Bernardini I, Rader D, Hoeg JM, Gahl WA (1991) Clinical and laboratory findings in the oculocerebrorenal syndrome of Lowe, with special reference to growth and renal function. N Engl J Med 324:1318–1325. https://doi.org/10.1056/NEJM199105093241904
De Matteis MA, Staiano L, Emma F, Devuyst O (2017) The 5-phosphatase OCRL in Lowe syndrome and Dent disease 2. Nat Rev Nephrol 13:455–470. https://doi.org/10.1038/nrneph.2017.83
Lowe CU, Terrey M, Mac LE (1952) Organic-aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am J Dis Child 83:164–184
Loi M (2006) Lowe syndrome. Orphanet J Rare Dis 1:16. https://doi.org/10.1186/1750-1172-1-16
Bokenkamp A, Ludwig M (2016) The oculocerebrorenal syndrome of Lowe: an update. Pediatr Nephrol 31:2201–2212. https://doi.org/10.1007/s00467-016-3343-3
Wrong OM, Norden AG, Feest TG (1994) Dent’s disease; a familial proximal renal tubular syndrome with low-molecular-weight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. QJM 87:473–493
Devuyst O, Thakker RV (2010) Dent’s disease. Orphanet J Rare Dis 5:28. https://doi.org/10.1186/1750-1172-5-28
Thakker RV (2000) Pathogenesis of Dent’s disease and related syndromes of X-linked nephrolithiasis. Kidney Int 57:787–793. https://doi.org/10.1046/j.1523-1755.2000.00916.x
Hoopes RR Jr, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ (2005) Dent disease with mutations in OCRL1. Am J Hum Genet 76:260–267. https://doi.org/10.1086/427887
Utsch B, Bokenkamp A, Benz MR, Besbas N, Dotsch J, Franke I, Frund S, Gok F, Hoppe B, Karle S, Kuwertz-Broking E, Laube G, Neb M, Nuutinen M, Ozaltin F, Rascher W, Ring T, Tasic V, van Wijk JA, Ludwig M (2006) Novel OCRL1 mutations in patients with the phenotype of Dent disease. Am J Kidney Dis 48(942):e941–e914. https://doi.org/10.1053/j.ajkd.2006.08.018
Cho HY, Lee BH, Choi HJ, Ha IS, Choi Y, Cheong HI (2008) Renal manifestations of Dent disease and Lowe syndrome. Pediatr Nephrol 23:243–249. https://doi.org/10.1007/s00467-007-0686-9
Sekine T, Nozu K, Iyengar R, Fu XJ, Matsuo M, Tanaka R, Iijima K, Matsui E, Harita Y, Inatomi J, Igarashi T (2007) OCRL1 mutations in patients with Dent disease phenotype in Japan. Pediatr Nephrol 22:975–980. https://doi.org/10.1007/s00467-007-0454-x
Shrimpton AE, Hoopes RR Jr, Knohl SJ, Hueber P, Reed AA, Christie PT, Igarashi T, Lee P, Lehman A, White C, Milford DV, Sanchez MR, Unwin R, Wrong OM, Thakker RV, Scheinman SJ (2009) OCRL1 mutations in Dent 2 patients suggest a mechanism for phenotypic variability. Nephron Physiol 112:27–36. https://doi.org/10.1159/000213506
Kaneko K, Hasui M, Hata A, Hata D, Nozu K (2010) Focal segmental glomerulosclerosis in a boy with Dent-2 disease. Pediatr Nephrol 25:781–782. https://doi.org/10.1007/s00467-009-1362-z
Solanki AK, Arif E, Morinelli T, Wilson RC, Hardiman G, Deng P, Arthur JM, Velez JC, Nihalani D, Janech MG, Budisavljevic MN (2018) A novel CLCN5 mutation associated with focal segmental glomerulosclerosis and podocyte injury. Kidney Int Rep 3:1443–1453. https://doi.org/10.1016/j.ekir.2018.06.003
Frishberg Y, Dinour D, Belostotsky R, Becker-Cohen R, Rinat C, Feinstein S, Navon-Elkan P, Ben-Shalom E (2009) Dent’s disease manifesting as focal glomerulosclerosis: is it the tip of the iceberg? Pediatr Nephrol 24:2369–2373. https://doi.org/10.1007/s00467-009-1299-2
Fervenza FC (2013) A patient with nephrotic-range proteinuria and focal global glomerulosclerosis. Clin J Am Soc Nephrol 8:1979–1987. https://doi.org/10.2215/cjn.03400313
Copelovitch L, Nash MA, Kaplan BS (2007) Hypothesis: Dent disease is an underrecognized cause of focal glomerulosclerosis. Clin J Am Soc Nephrol 2:914–918. https://doi.org/10.2215/cjn.00900207
van Berkel Y, Ludwig M, van Wijk JAE, Bökenkamp A (2017) Proteinuria in Dent disease: a review of the literature. Pediatr Nephrol 32:1851–1859. https://doi.org/10.1007/s00467-016-3499-x
Hichri H, Rendu J, Monnier N, Coutton C, Dorseuil O, Poussou RV, Baujat G, Blanchard A, Nobili F, Ranchin B, Remesy M, Salomon R, Satre V, Lunardi J (2011) From Lowe syndrome to Dent disease: correlations between mutations of the OCRL1 gene and clinical and biochemical phenotypes. Hum Mutat 32:379–388. https://doi.org/10.1002/humu.21391
Lee PT, Chou KJ, Fang HC (2012) Are tubular cells not only victims but also perpetrators in renal fibrosis? Kidney Int 82:128–130. https://doi.org/10.1038/ki.2012.120
Theilig F, Kriz W, Jerichow T, Schrade P, Hahnel B, Willnow T, Le Hir M, Bachmann S (2007) Abrogation of protein uptake through megalin-deficient proximal tubules does not safeguard against tubulointerstitial injury. J Am Soc Nephrol 18:1824–1834. https://doi.org/10.1681/asn.2006111266
Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV (2012) Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82:172–183. https://doi.org/10.1038/ki.2012.20
Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P (2002) A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13:630–638
Sarrab RM, Lennon R, Ni L, Wherlock MD, Welsh GI, Saleem MA (2011) Establishment of conditionally immortalized human glomerular mesangial cells in culture, with unique migratory properties. Am J Physiol Renal Physiol 301:F1131–F1138. https://doi.org/10.1152/ajprenal.00589.2010
Satchell SC, Tasman CH, Singh A, Ni L, Geelen J, von Ruhland CJ, O’Hare MJ, Saleem MA, van den Heuvel LP, Mathieson PW (2006) Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int 69:1633–1640. https://doi.org/10.1038/sj.ki.5000277
Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, Konstantakopoulos A, Lucocq J, Johannes L, Rabouille C, Greene LE, Lowe M (2005) Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell 16:3467–3479. https://doi.org/10.1091/mbc.E05-02-0120
Noakes CJ, Lee G, Lowe M (2011) The PH domain proteins IPIP27A and B link OCRL1 to receptor recycling in the endocytic pathway. Mol Biol Cell 22:606–623. https://doi.org/10.1091/UE10-08-0730
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310. https://doi.org/10.1002/aja.1002030302
Bierzynska A, McCarthy HJ, Soderquest K, Sen ES, Colby E, Ding WY, Nabhan MM, Kerecuk L, Hegde S, Hughes D, Marks S, Feather S, Jones C, Webb NJ, Ognjanovic M, Christian M, Gilbert RD, Sinha MD, Lord GM, Simpson M, Koziell AB, Welsh GI, Saleem MA (2017) Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney Int 91:937–947. https://doi.org/10.1016/j.kint.2016.10.013
Dogan RI, Getoor L, Wilbur WJ, Mount SM (2007) SplicePort—an interactive splice-site analysis tool. Nucleic Acids Res 35(Web Server issue):W285–W291. https://doi.org/10.1093/nar/gkm407
Yeo G, Burge CB (2004) Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol 11:377–394. https://doi.org/10.1089/1066527041410418
Reese MG, Eeckman FH, Kulp D, Haussler D (1997) Improved splice site detection in Genie. J Comput Biol 4:311–323. https://doi.org/10.1089/cmb.1997.4.311
Ramirez IB, Pietka G, Jones DR, Divecha N, Alia A, Baraban SC, Hurlstone AF, Lowe M (2012) Impaired neural development in a zebrafish model for Lowe syndrome. Hum Mol Genet 21:1744–1759. https://doi.org/10.1093/hmg/ddr608
Janne PA, Suchy SF, Bernard D, MacDonald M, Crawley J, Grinberg A, Wynshaw-Boris A, Westphal H, Nussbaum RL (1998) Functional overlap between murine Inpp5b and Ocrl1 may explain why deficiency of the murine ortholog for OCRL1 does not cause Lowe syndrome in mice. J Clin Invest 101:2042–2053. https://doi.org/10.1172/jci2414
Zhu X, Chen Z, Zeng C, Wang L, Xu F, Hou Q, Liu Z (2016) Ultrastructural characterization of the pronephric glomerulus development in zebrafish. J Morphol 277:1104–1112. https://doi.org/10.1002/jmor.20560
Billcliff PG, Noakes CJ, Mehta ZB, Yan G, Mak L, Woscholski R, Lowe M (2016) OCRL1 engages with the F-BAR protein pacsin 2 to promote biogenesis of membrane-trafficking intermediates. Mol Biol Cell 27:90–107. https://doi.org/10.1091/mbc.E15-06-0329
Swan LE, Tomasini L, Pirruccello M, Lunardi J, De Camilli P (2010) Two closely related endocytic proteins that share a common OCRL-binding motif with APPL1. Proc Natl Acad Sci U S A 107:3511–3516. https://doi.org/10.1073/pnas.0914658107
Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS (2001) CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol 159:2303–2308. https://doi.org/10.1016/s0002-9440(10)63080-5
Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P (2001) Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108:1621–1629. https://doi.org/10.1172/jci12849
Adair BD, Altintas MM, Moller CC, Arnaout MA, Reiser J (2014) Structure of the kidney slit diaphragm adapter protein CD2-associated protein as determined with electron microscopy. J Am Soc Nephrol 25:1465–1473. https://doi.org/10.1681/asn.2013090949
Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS (2003) CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300:1298–1300. https://doi.org/10.1126/science.1081068
Inoue K, Ishibe S (2015) Podocyte endocytosis in the regulation of the glomerular filtration barrier. Am J Physiol Renal Physiol 309:F398–F405. https://doi.org/10.1152/ajprenal.00136.2015
Soda K, Balkin DM, Ferguson SM, Paradise S, Milosevic I, Giovedi S, Volpicelli-Daley L, Tian X, Wu Y, Ma H, Son SH, Zheng R, Moeckel G, Cremona O, Holzman LB, De Camilli P, Ishibe S (2012) Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest 122:4401–4411. https://doi.org/10.1172/jci65289
Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS (1999) Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286:312–315
Kawachi H, Miyauchi N, Suzuki K, Han GD, Orikasa M, Shimizu F (2006) Role of podocyte slit diaphragm as a filtration barrier. Nephrology 11:274–281. https://doi.org/10.1111/j.1440-1797.2006.00583.x
Ovunc B, Otto EA, Vega-Warner V, Saisawat P, Ashraf S, Ramaswami G, Fathy HM, Schoeb D, Chernin G, Lyons RH, Yilmaz E, Hildebrandt F (2011) Exome sequencing reveals cubilin mutation as a single-gene cause of proteinuria. J Am Soc Nephrol 22:1815–1820. https://doi.org/10.1681/ASN.2011040337
Acknowledgements
The authors acknowledge the staff of the Bioimaging facility in Manchester for their support, the UK study of Nephrotic Syndrome and Hellyeh Hamidi for technical advice in experimental design. Finally, the authors acknowledge our colleagues in Bristol for generously providing the endothelial, mesangial and podocyte cell lines used.
Funding
R.P. is an Academic Clinical Fellow funded by the National Institute for Health Research (NIHR), and R.L and R.W.N are supported by a Wellcome Trust Senior Fellowship award (202860/Z/16/Z). M.L. is supported by a grant from the Lowe Syndrome Trust (ML/MU/2012). The authors received core funding from the Wellcome Trust (203128/Z/16/Z) to the Wellcome Trust Centre for Cell-Matrix Research at the University of Manchester, Manchester, UK. The authors also received funding from the NIHR, Kidney Research UK and the Lowe Syndrome Trust.
Author information
Authors and Affiliations
Contributions
R.L. and M.L. designed the study and R.P., R.W.N. and A.B. carried out the experiments; all authors analysed the data; R.P., R.W.N. and R.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Ethical approval was through the Manchester Renal Biobank reference: 16/NW/019.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Preston, R., Naylor, R.W., Stewart, G. et al. A role for OCRL in glomerular function and disease. Pediatr Nephrol 35, 641–648 (2020). https://doi.org/10.1007/s00467-019-04317-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04317-4