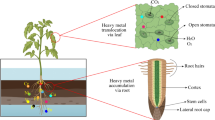
Abstract
The effects of S and Se treatment on cabbage, especially the interactions of S and Se metabolism with the biosynthesis of glucosinolate (GSL), including glucoraphanin, which is a major aliphatic GSL in cruciferous vegetables and the precursor of the anticancer compound sulforaphane, were examined. Cabbage plants were treated with sulfate and selenite (SeO32−), and the total S, Se, and GSL contents of cabbage head and outer foliage leaves were measured. Results showed that selenite treatment was beneficial to GSL biosynthesis and Se accumulation in cabbage head and outer foliage leaves. GSL synthesis was induced by exogenous selenite-elevated sulfate treatment at certain concentration ratios, i.e., 50-μΜ selenite + 1-mΜ sulfate or 100-μΜ selenite + 4-mΜ sulfate. A high exogenous sulfate concentration was more favorable to GSL accumulation than a low sulfate concentration. According to the relative expression of genes on GSL synthesis, an increase in the GSL content was attributed to the upregulation of gene expression and possible transportation from the outer foliage leaf to the head of cabbage. These results might be helpful for increasing the health benefits of cabbage by supplying exogenous S and Se. Further research should explore the effects of sulfate and selenite on GSL precursor substances to reveal the reason why total GSL contents increased.








Similar content being viewed by others
References
Anderson J (1993) Selenium interactions in sulfur metabolism. In: Kock D et al (eds) Sulfur nutrition and assimilation in higher plants: regulatory agricultural and environmental aspects. SPB Academic, The Hague
Barickman TC, Kopsell DA, Sams CE (2013) Selenium influences glucosinolate and isothiocyanates and increases sulfur uptake in Arabidopsis thaliana and rapid-cycling Brassica oleracea. J Agric Food Chem 61(1):202–209
Birringer M, Pilawa S, Flohé L (2002) Trends in selenium biochemistry. Nat Prod Rep 19(6):693–718
Cartea ME, Velasco P (2008) Glucosinolates in brassica foods: bioavailability in food and significance for human health. Phytochem Rev 7(2):213–229
Chen YC, Prabhu KS, Das A, Mastro AM (2013) Dietary selenium supplementation modifies breast tumor growth and metastasis. Int J Cancer 133(9):2054–2064
Clarke DB (2010) Glucosinolates, structures and analysis in food. Anal Methods 2(4):310–325
Clarke JD, Dashwood RH, Ho E (2008) Multi-targeted prevention of cancer by sulforaphane. Cancer Letters 269(2):291–304
Gandin V, Khalkar P, Braude J, Fernandes AP (2018) Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radical Biology & Medicine (on line)
Geuflores F, Nielsen MT, Nafisi M, Møldrup ME, Olsen CE, Motawia MS, Halkier BA (2009) Glucosinolate engineering identifies a gamma-glutamyl peptidase. Nat Chem Biol 5(8):575–577
Gigolashvili T, Yatusevich R, Berger B, Müller C, Flügge UI (2010) The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant J 51(2):247–261
Hirai MY, Sugiyama K, Sawada Y, Tohge T, Obayashi T, Suzuki A, Araki R, Sakurai N, Suzuki H, Aoki K (2007) Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc Natl Acad Sci U S A 104(15):6478–6483
Hsu FC, Wirtz M, Heppel SC, Bogs J, Krämer U, Khan MS, Bub A, Hell R, Rausch T (2011) Generation of Se-fortified broccoli as functional food: impact of Se fertilization on S metabolism. Plant Cell Environ 34(2):192–207
Huang K, Lin JC, Wu QY, Yan JY, Liu MY, Zhang S, Xiao WJ (2016) Changes in sulforaphane and selenocysteine methyltransferase transcript levels in broccoli treated with sodium selenite. Plant Molecular Biology Reporter 34:807–814
Khan MS, Hell R (2014) Applied cell biology of sulphur and selenium in plants. Springer, Berlin Heidelberg
Kim SJ, Ishii G (2010) Glucosinolate profiles in the seeds, leaves and roots of rocket salad (Eruca sativa Mill.) and anti-oxidative activities of intact plant powder and purified 4-methoxyglucobrassicin. Soil Sci Plant Nutr 52(3):394–400
Kim SJ, Kawaharada C, Jin S, Hashimoto M, Ishii G, Yamauchi H (2007) Structural elucidation of 4-(cystein-S-yl)butyl glucosinolate from the leaves of Eruca sativa. Journal of the Agricultural Chemical Society of Japan 71(1):114–121
Kim YB, Li XH, Kim SJ, Kim HH, Lee J, Kim H, Park SU (2013) MYB transcription factors Regulate glucosinolate biosynthesis in different organs of Chinese cabbage (Brassica rapa ssp. pekinensis). Molecules 18:8682–8695
Kraker JWD, Gershenzon J (2011) From amino acid to glucosinolate biosynthesis: protein sequence changes in the evolution of methylthioalkylmalate synthase in Arabidopsis. Plant Cell 23(1):38–53
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Lv J, Wu J, Zuo J, Fan L, Shi J, Gao L, Li M, Wang Q (2017) Effect of Se treatment on the volatile compounds in broccoli. Food Chemistry 216:225–233
Lyi SM, Heller LI, Rutzke M, Welch RM, Kochian LV, Li L (2005) Molecular and biochemical characterization of the selenocysteine Se-methyltransferase gene and Se-methylselenocysteine synthesis in broccoli. Plant Physiology 138(1):409–420
Mahn A (2017) Modelling of the effect of selenium fertilization on the content of bioactive compounds in broccoli heads. Food Chemistry 233:492–499
Malagoli M, Schiavon M, Dall’Acqua S, Pilonsmits EAH (2015) Effects of selenium biofortification on crop nutritional quality. Front Plant Sci 6(280):1–5
Matich AJ, Mckenzie MJ, Lill RE, Brummell DA, Mcghie TK, Chen KY, Rowan DD (2012) Selenoglucosinolates and their metabolites produced in Brassica spp. fertilised with sodium selenate. Phytochemistry 75(3):140–152
Matusheski NV, Juvik JA, Jeffery EH (2004) Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 65(9):1273–1281
Mullineaux PM, Rausch T (2005) Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynthesis Research 86(3):459–474
Pérez C, Barrientos H, Román J, Mahn A (2014) Optimization of a blanching step to maximize sulforaphane synthesis in broccoli florets. Food Chem 145(7):264–271
Pu Q, Shi S, Zhang L, Gao Q, Ren X, Xiang C, Yang P, Lin B, Geng M (2017) Cloning and expression analysis of BoIAA2 and BoIAA19 genes of AUX/IAA family in cabbage. J Agric Sci Technol 19(9):24–33
Pyrzynska K (2009) Selenium speciation in enriched vegetables. Food Chem 114:1183–1191
Rausch T, Wachter A (2005) Sulfur metabolism: a versatile platform for launching defence operations. Trends in Plant Science 10(10):503–509
Rosen CJ, Fritz VA, Gardner GM, Hecht SS, Carmella SG, Kenney PM (2005) Cabbage yield and glucosinolate concentrations as affected by nitrogen and sulfur fertility. HortScience 40:1493–1498
Sams CE, Panthee DR, Charron CS, Kopsell DA, Yuan JS (2011) Selenium regulates gene expression for glucosinolate and carotenoid biosynthesis in arabidopsis. Journal of the American Society for Horticultural Science American Society for Horticultural Science 136(1):23–34
Sang JP, Minchinton IR, Johnstone PK, Rjw T (1984) Glucosinolate profiles in the seed, root and leaf tissue of cabbage, mustard, rapeseed, radish and swede. Can J Plant Sci 64(1):77–93
Sepúlveda I, Barrientos H, Mahn A, Moenne A (2013) Changes in SeMSC, glucosinolates and sulforaphane levels, and in proteome profile in broccoli (Brassica oleracea var. Italica) fertilized with sodium selenate. Molecules 18(5):5221–5234
Sønderby IE, Geu-Flores F, Halkier BA (2010) Biosynthesis of glucosinolates--gene discovery and beyond. Trends in Plant Science 15(5):283–290
Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynthesis Research 86(3):373–389
Sors TG, Ellis DR, Na GN, Lahner B, Lee S, Leustek T, Pickering IJ, Salt DE (2010) Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant Journal for Cell & Molecular Biology 42(6):785–797
Sun J, Zhang M, Chen P (2016) GLS-Finder: a platform for fast profiling of glucosinolates in brassica Vegetables. J Agric Food Chem 64(21):4407–4415
Tarun AS, Böck A, Caruso J, Terry N (2004) Overexpression of selenocysteine methyltransferase in Arabidopsis and Indian mustard increases selenium tolerance and accumulation. Plant Physiology 135(1):377–383
Tian M, Xu X, Liu Y, Xie L, Pan S (2016) Effect of Se treatment on glucosinolate metabolism and health-promoting compounds in the broccoli sprouts of three cultivars. Food Chemistry 190:374–380
Tian M, Yang Y, Avila FW, Fish T, Yuan H, Hui M, Pan S, Thanhauser T, Li L (2018) Effects of selenium supplementation on glucosinolate biosynthesis in broccoli. Journal of Agricultural & Food Chemistry 66(30):8036–8044
Valdez-Barilla JR, Quinn CF, Pilon-Smits EAH (2011) Selenium accumulation in plants – phytotechnological applications and ecological implications. International journal of phytoremediation 13:166–178
Zhao FJ (2010) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytologist 178(1):92–102
Acknowledgments
This research was funded by National Natural Science Foundation of China (No. 31772325, 31902023), Hunan Provincial Natural Science Foundation of China (2018JJ3217), Hunan Provincial Sci-Tech Project (2018NK2022), Open Foundation of Key laboratory of Biology and Genetic Improvement of Horticultural Crops, Ministry of Agriculture (IVF201702), and Foundation for Young Scholars of Hunan Agricultural University(17QN33).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key message
Selenite treatment was beneficial to glucosinolate (GSL) biosynthesis and Se accumulation in cabbage head and outer foliage leaf. GSLs synthesis was induced by exogenous selenite-elevated sulfate treatment of 50 mΜ selenite +1 mΜ sulfate or 100 mM selenite +4 mΜ sulfate.
Rights and permissions
About this article
Cite this article
Wang, J., Mao, S., Xu, H. et al. Effects of Sulfur and Selenium on Glucosinolate Biosynthesis in Cabbage. Plant Mol Biol Rep 38, 62–74 (2020). https://doi.org/10.1007/s11105-019-01178-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-019-01178-x