Abstract
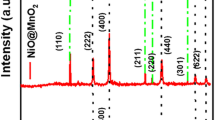
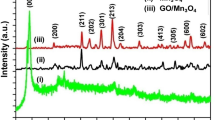
Pure Mn3O4 and Mn3O4/rGO hybrid nanocomposites were synthesized by sol–gel based in situ reduction method. The structural properties of pure and nanocomposite materials were studied by XRD. The crystallite size of the Mn3O4 nanoparticle was reduced in the nanocomposite as observed by XRD analysis. SEM and TEM images depict the spherical morphology of pristine Mn3O4 nanoparticles and decoration of Mn3O4 nanoparticle on rGO sheets. Raman spectra confirm the formation of Mn3O4/rGO nanohybrid composites as the Ag mode of Mn3O4, D, and G bands of rGO were observed in the spectra. FTIR spectra confirm the presence of various functional groups of GO and the in situ reduction of GO into rGO. The electrochemical properties of the Mn3O4 and Mn3O4/rGO composites were investigated by cyclic voltammetry analysis using 1 M KOH as an electrolyte. The cyclic voltammetry results show the pseudocapacitance behavior of Mn3O4, whereas the hybrid nanocomposite exhibits the combined behaviors of pseudocapacitance and EDLC. The chronopotentiometry analysis demonstrated that the specific capacitance of Mn3O4/rGO nanocomposite (427 F g−1) was relatively higher than that of Mn3O4 (136 F g−1) at 1 A/g. The impact of KOH electrolyte over the specific capacitance of a electrode material was comparatively analyzed with different electrolytes. The enhancement in the specific capacitance of the nanohybrid composite was attributed due to the strong electrode–electrolyte interaction of hybrid electrode material and synergetic effect of Mn3O4 and rGO.

Highlights
-
Mn3O4/rGO nanocomposite was synthesized by sol–gel based in-situ reduction method.
-
Crystallite size of the Mn3O4 was reduced consistently while adding rGO 2 and 5%.
-
TEM image confirms the spherical morphology of the Mn3O4 nanoparticle (~10 nm) and Raman spectroscopy results confirm the formation of composites.
-
Specific capacitance of the Mn3O4/rGO nanocomposite (427 F g−1) was higher than Mn3O4 (136 F g−1).
-
High specific capacitance of composite is due to the synergetic effect of Mn3O4/rGO.










Similar content being viewed by others
References
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854. https://doi.org/10.1038/nmat2297
González A, Goikolea E, Barrena JA, Mysyk R (2016) Review on supercapacitors: technologies and materials. Renew Sustain Energy Rev 58:1189–1206. https://doi.org/10.1016/j.rser.2015.12.249
Reddy ALM, Gowda SR, Shaijumon MM, Ajayan PM (2012) Hybrid nanostructures for energy storage applications. Adv Mater 24:5045–5064. https://doi.org/10.1002/adma.201104502
Bose VC, Biju V (2015) Mixed valence nanostructured Mn3O4 for supercapacitor applications. Bull Mater Sci 38:865–873. https://doi.org/10.1007/s12034-015-0906-z
Shah HU, Wang F, Toufiq AM, Ali S, Khan ZUH, Li Y, Hu J, He K (2018) Electrochemical properties of controlled size Mn3O4 nanoparticles for supercapacitor applications. J Nanosci Nanotechnol 18:719–724. https://doi.org/10.1166/jnn.2018.14644
Sun Y, Zhang W, Li D, Gao L, Hou C, Zhang Y, Liu Y (2015) Facile synthesis of MnO2/rGO/Ni composite foam with excellent pseudocapacitive behavior for supercapacitors. J Alloy Compd 649:579–584. https://doi.org/10.1016/j.jallcom.2015.07.212
Lee JW, Hall AS, Kim J-D, Mallouk TE (2012) A facile and template-free hydrothermal synthesis of Mn3O4 nanorods on graphene sheets for supercapacitor electrodes with long cycle stability. Chem Mater 24:1158–1164. https://doi.org/10.1021/cm203697w
Sankar KV, Kalpana D, Selvan RK (2012) Electrochemical properties of microwave-assisted reflux-synthesized Mn3O4 nanoparticles in different electrolytes for supercapacitor applications. J Appl Electrochem 42:463–470. https://doi.org/10.1007/s10800-012-0424-2
Wang B, Park J, Wang C, Ahn H, Wang G (2010) Mn3O4nanoparticles embedded into graphene nanosheets: preparation, characterization, and electrochemical properties for supercapacitors. Electrochim Acta 55:6812–6817. https://doi.org/10.1016/j.electacta.2010.05.086
Ravichandran K, Praseetha PK, Arun T, Gobalakrishnan S (2018). Synthesis of nanocomposites. In Synthesis of Inorganic Nanomaterials. (pp. 141–168). Woodhead Publishing, Cambridge
Sengottaiyan C, Jayavel R, Shrestha RG, Hill JP, Ariga K, Shrestha LK (2017) Electrochemical supercapacitance properties of reduced graphene oxide/Mn2O3:Co3O4 nanocomposite. J Inorg Organomet Polym Mater 27:576–585. https://doi.org/10.1007/s10904-017-0501-4
Cai LN, Guo Y, Lu AH, Branton P, Li WC (2012) The choice of precipitant and precursor in the co-precipitation synthesis of copper manganese oxide for maximizing carbon monoxide oxidation. J Mol Catal A Chem 360:35–41. https://doi.org/10.1016/j.molcata.2012.04.003
Hu Y, Guan C, Feng G, Ke Q, Huang X, Wang J (2015) Flexible asymmetric supercapacitor based on structure-optimized Mn3O4/reduced graphene oxide nanohybrid paper with high energy and power density. Adv Funct Mater 25:7291–7299. https://doi.org/10.1002/adfm.201503528
Nagamuthu S, Vijayakumar S, Muralidharan G (2013) Synthesis of Mn3O4/amorphous carbon nanoparticles as electrode material for high performance supercapacitor applications. Energy Fuels 27:3508–3515. https://doi.org/10.1021/ef400212b
Raj BGS, Ramprasad RNR, Asiri AM, Wu JJ, Anandan S (2015) Ultrasound assisted synthesis of Mn3O4 nanoparticles anchored graphene nanosheets for supercapacitor applications. Electrochim Acta 156:127–137. https://doi.org/10.1016/j.electacta.2015.01.052
Yang H, Jiang J, Zhou W, Lai L, Xi L, Lam Y, Shen Z, Khezri B, Yu T (2011) Influences of graphene oxide support on the electrochemical performances of graphene oxide-MnO2 nanocomposites. Nanoscale Res Lett 6:531. https://doi.org/10.1186/1556-276X-6-531
Liu Z, Zhang L, Xu G, Zhang L, Jia D, Zhang C (2017) Mn3O4 hollow microcubes and solid nanospheres derived from a metal formate framework for electrochemical capacitor applications. RSC Adv 7:11129–11134. https://doi.org/10.1039/C7RA00435D
Wu Y, Liu S, Wang H, Wang X, Zhang X, Jin G (2013) A novel solvothermal synthesis of Mn3O4/graphene composites for supercapacitors. Electrochim Acta 90:210–218. https://doi.org/10.1016/j.electacta.2012.11.124
Dubal DP, Dhawale DS, Salunkhe RR, Fulari VJ, Lokhande CD (2010) Chemical synthesis and characterization of Mn3O4 thin films for supercapacitor application. J Alloy Compd 497:166–170. https://doi.org/10.1016/j.jallcom.2010.02.182
Ozoemena KI, Raju K, Ejikeme PM, Ozoemena KI (2017) High-performance Mn3O4/onion-like carbon (OLC) nanohybrid pseudocapacitor: unravelling the intrinsic properties of OLC against other carbon supports. Carbon NY 117:20–32. https://doi.org/10.1016/j.carbon.2017.02.050
Yang X, He Y, Bai Y, Zhang J, Kang L, Xu H, Shi F, Lei Z, Liu ZH (2016) Mn3O4 nanocrystalline/graphene hybrid electrode with high capacitance. Electrochim Acta 188:398–405. https://doi.org/10.1016/j.electacta.2015.12.024
G An, P Yu, M Xiao, Z Liu, Z Miao, K Ding, L Mao (2008) Low-temperature synthesis of Mn3O4 nanoparticles loaded on multi-walled carbon nanotubes and their application in electrochemical capacitors. Nanotechnology. 19. https://doi.org/10.1088/0957-4484/19/27/275709
Zhu L, Zhang S, Cui Y, Song H, Chen X (2013) One step synthesis and capacitive performance of graphene nanosheets/Mn3O4 composite. Electrochim Acta 89:18–23. https://doi.org/10.1016/j.electacta.2012.10.157
Karuppaiah M et al. (2019) Solvent dependent morphological modification of micro-nano assembled Mn2O3/NiO composites for high performance supercapacitor applications. Ceram Int 45.4:4298–4307
Eigler S, Dimiev AM (2017). Functionalization and reduction of graphene oxide, In Graphene Oxide: Fundamentals and applications. (pp. 175–229). John Wiley & Sons, NJ
Sengottaiyan C, Jayavel R, Shrestha RG, Subramani T, Maji S, Kim JH, ... Shrestha LK (2018). Indium Oxide/Carbon Nanotube/Reduced Graphene Oxide Ternary Nanocomposite with Enhanced Electrochemical Supercapacitance. Bull Chem Soc Jpn 92:521–528
Acknowledgements
The work was financially supported by DST-Nanomission under M.Tech Program (SR/NM/PG-02/2015), DST-SERB under ECR award (ECR/2015/000575), and DST-SERB under EMR (EMR/2016/007550). The author (SH) thank DST nanomission for the MTech, student Fellowship (2015–2017). The author (MMI) thankful to Anna University for the Anna Centenary Research Fellowship (CRF/ACRF/2018/AR1/50).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamed Ismail, M., Hemaanandhan, S., Mani, D. et al. Facile preparation of Mn3O4/rGO hybrid nanocomposite by sol–gel in situ reduction method with enhanced energy storage performance for supercapacitor applications. J Sol-Gel Sci Technol 93, 703–713 (2020). https://doi.org/10.1007/s10971-019-05184-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05184-z