Abstract
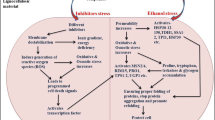
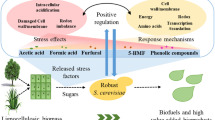
S.cerevisiae is an industrially important organism known for its ability to produce ethanol as the demand for ethanol is increasing day by day all over the world, the need to find better and alternative ways to increase ethanol production is also rising. In this work we have proposed such alternative but effective method for producing ethanol by S.cerevisiae. Here, we are reporting for the first time the effect of nitrosative stress on ethanol production. Under in vivo condition, nitrosative stress is marked by the modification of macromolecules in the presence of reactive nitrogen species (RNS). Our result showed that treated cells were more capable for ethanol production compared with untreated cells. Our result also showed enhanced alcohol dehydrogenase activity under stressed condition. Further ethanol production was also optimized by using Response Surface Methodology (RSM) with stressed cells. Further, production of ethanol with immobilized beads of stress affected Saccharomyces cerevisiae was also determined. Overall, the obtained data showed that under nitrosative stress, the maximum ethanol production is 34.4 g/l after 24 h and such higher production was observed even after several cycles of fermentation. This is the first report of this kind showing the relation between nitrosative stress and ethanol production in Saccharomyces cerevisiae which may have important industrial application.








Similar content being viewed by others
References
Willaert, R. G. (2017). Yeast biotechnology. Fermentation, 3, 7–10.
Mohd Azhar, S. H., Abdullah, R., & Jambo, S. A. et al. (2017). Yeasts in sustainable bioethanol production: a review. Biochemistry and Biophysics Reports, 10, 52–61.
Verbelen, P. J., De Schutter, D. P., & Delvaux, F. et al. (2006). Immobilized yeast cell systems for continuous fermentation applications. Biotechnology Letters, 28, 1515–1525.
Zaldivar, J., Nielson, J., & Olsson, L. (2001). Fuel ethanol production from lignocelluloses: a challenge for metabolic engineering and process integration. Applied Microbiology and Biotechnology, 56, 17–34.
Nevoigt, E. (2008). Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiology and Molecular Biology Reviews, 72, 379–412.
Matallana, E., & Aranda, A. (2017). Biotechnological impact of stress response on wine yeast. Letters in Applied Microbiology, 64, 103–110.
Pretorius, I. S. (2000). Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast, 16, 675–729.
Forman, H., Fukuto, J. M., & Miller, T. (2008). The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Archives of Biochemistry and Biophysics, 477, 183–195.
Palmer, R. M., Rees, D. D., & Ashton, D. S. (1980). L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochemical and Biophysical Research Communications, 153, 1251–1256.
Kurutas, E. B. (2016). The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutrition Journal, 15, 71 https://doi.org/10.1186/s12937-016-0186-5.
Bhattacharjee, A., Majumdar, U., & Maity, D. et al. (2010). Characterizing the effect of nitrosative stress in Saccharomyces cerevisiae. Archives of Biochemistry and Biophysics, 496, 109–116.
Rogstam, A., Larsson, J. T., & Kjelgaard, P. (2007). Mechanisms of adaptation to nitrosative stress in Bacillus subtilis. Journal of Bacteriology, 189, 3063–3071.
Fang, F. C. (2004). Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nature Reviews Microbiology, 2, 820–832.
Bhattacharjee, A., Majumdar, U., & Maity, D. et al. (2009). In vivo protein tyrosine nitration in S. cerevisiae: Identification of tyrosine-nitrated proteins in mitochondria. Biochemical and Biophysical Research Communications, 388, 612–617.
Crow, J. P., Beckman, J. S., & McCord, J. M. (1995). Sensitivity of the essential zinc-thiolate moiety of yeast alcohol dehydrogenase to hypochlorite and peroxynitrite. Biochemistry, 34, 3544–3552.
Claudia, L., Nataliya, L., & Müller, A. (2013). Redox proteomics uncovers peroxynitrite-sensitive proteins that help Escherichia coli to overcome nitrosative stress. The Journal of Biological Chemistry, 288, 19698–19714.
Hagman, A., Sa¨ll, T., & Compagno, C., et al. (2013). Yeast ‘make-accumulate-consume’’ life strategy evolved as a mMulti-step process that predates the wholeg duplication. PLoS ONE, 8, e68734.
Sayyad, S. F., Chaudhari, S. R., & Panda, B. P. (2015). Quantitative determination of ethanol in arishta by using UVvisible spectrophotometer. Pharmaceutical and Biological Evaluations, 2, 204–207.
Zakya, A. S., Pensupa, N., & Andrade-Eiroa, Á. (2017). A new HPLC method for simultaneously measuring chloride, sugars, organic acids and alcohols in food samples. Journal of Food Composition and Analysis, 56, 25–33.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Richard, L. T. (1998). The competitive inhibition of yeast alcohol dehydrogenase by 2,2,2-trifluoroethanol. Biochemical Education, 26, 239–242.
Pereira, A. P., Estevinho, L. M., & Mendes-Faia, A. (2014). Mead production: fermentative performance of yeasts entrapped in different concentrations of alginate. Journal of the Institute Brewing, 120, 575–580.
Saha, S. P., & Ghosh, S. (2014). Optimization of xylanase production by Penicillium citrinum xym2 and application in saccharification of agro-residues. Biocatalysis and Agricultural Biotechnology, 3, 188–196.
Dhawan, V. (2014). Reactive oxygen and nitrogen species: general considerations. In N. K. Ganguly, S. K. Jindal, S. Biswal, P. J. Barnes & R. Pawankar Eds., Studies on Respiratory Disorders (pp. 27–47). New York, NY: Humana Press.
Staab, C. A., Alander, J., & Brandt, M. (2008). Reduction of S- nitroglutathione by alcohol dehydrogenase III is faciliated by substrate alcohols via direct cofactor recycling and leads to GSH- controlled formation of glutathione transferase inhibitors. Biochemical Journal, 413, 493–504.
Gow, A. J., & Stamler, J. S. (1998). Reaction between nitric oxide and haemoglobin under physiological condition. Nature, 391, 169–173.
Sahoo, R., Bhattacharjee, A., & Majumder, U. et al. (2009). A novel role of catalase in detoxificationo peroxinitrite in Saccharomyces cerevisiae. Biochemical and Biophysical Research Communications, 38, 5507–5511.
Liu, L., Hausladen, A., & Zeng, M., et al. (2001). A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature, 410, 490–494.
Acknowledgements
The authors acknowledge the University of North Bengal for providing essential infrastructure and financial support to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sengupta, S., Deb, M., Nath, R. et al. Optimization of Ethanol Production using Nitrosative Stress Exposed S.cerevisiae. Cell Biochem Biophys 78, 101–110 (2020). https://doi.org/10.1007/s12013-019-00897-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-019-00897-y