Abstract
Recent reviews of transcranial direct current stimulation (tDCS) show limited support for its initially cited enhancing effects on working memory (WM). They highlight the need for additional research, assessing the specific circumstances that optimize stimulation outcome. Social stress is an attractive candidate in this regard, as it affects WM and is mediated by prefrontal cortex activity; tDCS that targets these neuronal networks may, therefore, interact with social stress to affect WM. Our objective was to explore the interaction between social stress and tDCS on WM performance in a healthy cohort, 69 female participants were randomized to four experimental conditions (i.e., 2 × 2 design): stimulation (dlPFC tDCS vs. sham stimulation) and stress manipulation (Trier Social Stress Test [TSST] procedure vs. a friendly control TSST). Participants’ attention, WM (assessed using an n-back task), and subjective/objective indicators of stress were assessed. A significant Stimulation × Stress Manipulation interaction was found, F(1, 65) = 6.208, p = .015, suggesting that active tDCS may increase WM performance in the no-stress conditions, while decreasing it under stress. Follow-up analyses of variance, however, were not significant (i.e., ps=.083 / .093), and Bayesian analyses were inconclusive. In conclusion, stress seems to be a crucial factor in determining the effects of tDCS, and tDCS may have an enhancing effect on WM at lower levels of stress, while being detrimental at higher stress levels (i.e., reversing the direction of effect). Possible theoretical underpinnings of the findings are discussed, while acknowledging the need for further research.
Similar content being viewed by others
Transcranial direct current stimulation (tDCS) is a noninvasive, safe, and easy-to-use technique for modifying cerebral excitability through electrodes attached to distinct areas on the skull (Brunoni et al., 2012; Nitsche et al., 2008; Schutter & Wischnewski, 2016). It applies a weak direct current that does not produce immediate action potentials, but rather modulates the resting potential of cortical neurons. It thereby modifies their responsiveness to endogenous stimuli. Significant tDCS effects were reported on motor, visual, somatosensory, vestibular, cognitive, and emotional functioning (Brunoni et al., 2013; Coffman, Clark, & Parasuraman, 2014; Lapenta, Valasek, Brunoni, & Boggio, 2014; Priori, Berardelli, Rona, Accornero, & Manfredi, 1998; Utz, Dimova, Oppenländer, & Kerkhoff, 2010).
Executive attention is a core component of WM, maintaining memory representations in a highly active state in the presence of interferences (Kane & Engle, 2002). The dorsolateral prefrontal cortex (dlPFC) is involved in tasks, such as the n-back task, which require WM operations that rely heavily on executive attention (Kane & Engle, 2002; LaBar & Cabeza, 2006). tDCS over the PFC, and more specifically over the dlPFC, was found to enhance WM performance (Fregni, Boggio, Nitsche, Rigonatti, & Pascual-Leone, 2006; Meiron & Lavidor, 2013; Zaehle, Sandmann, Thorne, Jäncke, & Herrmann, 2011). Recent systematic reviews and meta-analyses, however, suggest that these initial findings consisted of either small/nonsignificant effects, or failed to be replicated (Berryhill, Peterson, Jones, & Stephens, 2014; Hill, Fitzgerald, & Hoy, 2016; Horvath, Carter, & Forte, 2014; Mancuso, Ilieva, Hamilton, & Farah, 2016). This has somewhat curbed the enthusiasm regarding the potential enhancing effects of tDCS and raised the need to study the specific conditions that either facilitate or interfere with its impact on performance.
One of the factors that may modulate tDCS effects on WM is stress, the subjective experience that one’s available resources are not sufficient to cope with the situational demands (Folkman, 2013; Lazarus & Folkman, 1984). Stress is a key element in clinical research that is often neglected in tDCS studies performed in the laboratory. This is unfortunate, as it was found that cognitive functions—and specifically WM—are negatively affected by stress, probably via its influence on the PFC (Arnsten, 2009, 2015; Kudielka, Hellhammer, & Kirschbaum, 2007). For example, Schoofs, Preuß, and Wolf (2008) showed that the Trier Social Stress Test (TSST), a well-documented task that affects both subjective and physiological markers of stress, impairs WM (see also Dickerson & Kemeny, 2004; Kirschbaum, Pirke, & Hellhammer, 1993; Kudielka et al., 2007). At the same time, tDCS to the PFC was effective in modulating the stress response to TSST (Antal et al., 2014). Such findings are not surprising considering the key role of the PFC in regulating affect and stress. Both tDCS and stress, therefore, converge on neuronal networks that affect WM (i.e., dlPFC executive neural networks and their association with the hippocampus; Bremner, 1999; Kane & Engle, 2002; M. Schneider et al., 2017). Together, these findings suggest that studying the interactive effects of tDCS and stress is of value.
The current study assessed the possible interaction between dlPFC tDCS and social stress on WM performance. This was achieved using a 2 × 2 design, with stimulation (tDCS vs. sham stimulation) and a TSST-based stress manipulation (STRESS vs. noStress) serving as independent variables. An n-back task, sensitive to executive dlPFC activity (Kane & Engle, 2002; Meiron & Lavidor, 2013), was used to assess WM performance and served as the dependent variable. We hypothesized that tDCS will enhance WM when performed in a control, no-stress condition (based on Meiron & Lavidor, 2013). Stress (without stimulation), in contrast, was hypothesized to have a deleterious effect on WM (based on Schoofs et al., 2008). Finally, we hypothesized that tDCS would mitigate the effect of stress (based on Antal et al., 2014), including stress-induced WM impairment. These hypotheses were made based on previous findings, though some were made more tentatively considering the scarcity of previous research (i.e., hypotheses regarding the interactive effect of tDCS and stress).
Method
Participants
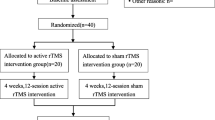
Healthy adults (i.e., undergraduate students who received course credit for participating in the study) were assessed for eligibility (n = 123). Stratified randomization (controlling for gender proportion in each condition) was performed by the university’s online experiment allocation system, after candidates indicated their eligibility based on the study’s inclusion/exclusion criteria (n = 106). Inclusion criteria were that they were healthy, right-handed, native Hebrew speakers, and had normal/corrected-to-normal vision. Candidates were excluded if they had metallic implants, skin disease, past/present neurological disorders (including a learning disability), substance use disorder, blood pressure problems, or were pregnant/potentially pregnant at the time of the study. The resulting sample consisted of 75.50% females (group differences ns), χ23= 0.947, p = .814, with an average age of 23.87 ± 1.79 (group differences ns), F(3, 102) = 1.416, p = .242. The inclusion/exclusion criteria were more thoroughly assessed during the experimental meeting, after obtaining written informed consent. Consequently, six participants were excluded from the study after randomization (i.e., due to learning disabilities or substance use). See CONSORT diagram in Fig. 1.
One hundred participants began the experimental procedure. One participant intended to undergo sham stimulation, but felt ill at the start of the experiment, and his participation in the study was discontinued. The participation of five additional participants was discontinued due to technical problems. Overall, 94 participants completed the experimental procedure. The data of the additional participants were excluded from analyses due to the following reasons: (a) Extremely poor performance in the cognitive tasks (i.e., below chance level), raising the possibility that they did not exert sufficient effort in performing the tasks (n = 3). (b) The male representation in the final sample was too low to allow between-group comparison (e.g., n = 3 in the tDCS+noStress group). Because of known gender differences in response to stress and tDCS (Bell, Willson, Wilman, Dave, & Silverstone, 2006; Goldstein et al., 2005; Kudielka et al., 2007; Meiron & Lavidor, 2013; Schoofs et al., 2008; Wolf, 2006) and to minimize within-group variability, the data of male participants were excluded from the analyses. Overall, the data of 69 female participants were analyzed (see Fig. 1).
The study was approved by the university’s ethics committee and was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Materials
Stimulation
Stimulation was delivered by a battery-driven constant current stimulator (Chattanooga Ionto, Iontophoresis System, Hixson, TN, USA), using a pair of saline-soaked synthetic sponge electrodes. Electrode size was 5 × 5 cm. Following Meiron and Lavidor (2013), the electrode montage was Cz for the reference cathode (according to the 10–20 international system for EEG electrode placement) and the right dlPFCFootnote 1 for the anode (corresponding to F4/AF4 in the 10–20 system). The stimulation site was located according to a previously published procedure for optimal localization of the dlPFC (Fitzgerald, Maller, Hoy, Thomson, & Daskalakis, 2009). When delivering active tDCS, the current was applied for 20 minutes with a fade-in/fade-out ramp of 30 seconds. The current intensity was 2.0 mA. When delivering sham stimulation, the same fade-in/fade-out ramp was applied, but the constant current lasted only 30 seconds. Because we employed a single-blind paradigm, all participants were informed that they were receiving active stimulation.
Working memory task
In the original n-back task, stimuli are consecutively presented, and the participant is expected to respond only if the presented stimulus matches the one presented n steps earlier (Kirchner, 1958). The task has variations that differ in the number of n steps, stimuli type (e.g., pictures, letters, words), and so on (e.g., Jaeggi et al., 2010). In the current study, a modified verbal two-back task, reported to engage WM and executive attention circuits in the dlPFC, was employed (Meiron & Lavidor, 2013). In this task, a series of word pairs are presented. When a single word unexpectedly appears and it matches one of the words in a pair that appeared two steps earlier, the participant should respond by a key press.Footnote 2 This go/no-go response modality increases the task’s sensitivity to the executive component of WM, associated with response inhibition. Participants in all study groups performed an identical WM task, with accuracy (% correct responses) and reaction time for correct responses (ms) serving as outcome measures (reaction time <200 ms were considered as outliers and discarded prior to analyses; similar to Schmiedek, Oberauer, Wilhelm, Suss, & Wittmann, 2007). The task’s duration is 12.5 min (i.e., four rounds of 2.5 minutes with resting intervals of 30 seconds before and after each round). A detailed description of the task and its neuropsychological correlates can be found in previous publications (Meiron & Lavidor, 2013, 2014).
Baseline attention task
The Identification task from the CogState Brief Battery (Lim et al., 2013) assessed possible baseline group differences in visual attention. This computerized task measures attention using a choice reaction-time paradigm (3-minute duration). A playing card is presented facedown in the center of the screen. As soon as it flips over, the participant must press “yes” if the card is red and “no” if the color differs from red. The participant is requested to respond as quickly as possible, while maintaining a high level of accuracy.
Cortisol level
Cortisol samples were collected from a random subset of 40 participants (10 participants in each group), as an objective stress indicator (i.e., validation of the stress-manipulation effect). Four of these participants, however, did not have valid saliva samples for all three time points, and their data were therefore not included in the analyses (see Fig. 1). Saliva was collected using Salivette collection devices (Sarstedt, Nuembrecht, Germany). Free cortisol levels were measured using a cortisol ELISA immunoassay kit (Salimetrics).
Self-report stress questionnaires
(a) State-Trait Anxiety Inventory (STAI): The STAI is one of the most commonly used self-report questionnaires for measuring anxiety (Spielberger, Gorsuch, & Lushene, 1970). It is a 20-item inventory, composed of short verbal statements (i.e., emotional states) that the participant rates on a 4-point Likert-type scale (higher scores indicating higher anxiety levels). The STAI has good psychometric qualities, with Cronbach’s α varying between 0.86 to 0.95 (Rossi & Pourtois, 2012). As part of the current study, the STAI-T assessed the participants’ stress predisposition at the beginning of the trial (baseline), while the STAI-S measured their transitory anxiety levels before and after the experimental manipulations. (b) Visual Analogue Scale (VAS): A VAS measured subjective stress before and after the experimental manipulations (Gaab, Rohleder, Nater, & Ehlert, 2005).
Procedure
General
Participants were requested to abstain from eating, drinking, or smoking 1 hour prior to the experimental session. Willing candidates signed a written informed consent form, filled the STAI-T, performed the CogState identification task (i.e., attention task), and underwent the first cortisol saliva test (t1). Following dlPFC localization, participants received 20 minutes of either active or sham unilateral stimulation to the right dlPFC (termed tDCS and shamStimulation, respectively). Ten minutes after starting stimulation (allowing time to familiarize with any somatosensory stimulation-related sensations), the participants performed the practice stage of the n-back task (2 minutes duration).Footnote 3 Following stimulation, the participants completed the second battery of questionnaires (STAI-S and VAS) and underwent the second saliva test (t2). Next, they underwent the TSST or its control condition (termed STRESS and noStress, respectively) and performed the n-back task (elaborated in the following subsection). Finally, they performed the third battery of questionnaires (STAI-S and VAS) and underwent the third cortisol saliva test (t3). The participants were then debriefed. All testing sessions were conducted between 10:00 a.m. and 12:30 p.m., to avoid circadian cortisol variation (Schoofs et al., 2008). Figure 2 details the study procedure.
Stress manipulation
The Trier Social Stress Test (TSST) is a well-validated stress manipulation (e.g., Frisch, Häusser, & Mojzisch, 2015; Kudielka et al., 2007), in which a job interview is simulated. It was found to affect hypothalamic–pituitary–adrenal (HPA) axis activity (Kirschbaum et al., 1993). More specifically, cortisol levels increase up to twofold from baseline in 70% to 80% of participants, with a first peak 5–10 minutes after the end of the TSST and another one after about 20 minutes (Allen, Kennedy, Cryan, Dinan, & Clarke, 2014; Kudielka et al., 2007). Additional stress indicators that are affected by the TSST are α-amylase, catecholamines, growth hormone, prolactin, testosterone, as well as various cardiovascular parameters (Frisch et al., 2015; Kudielka et al., 2007). Finally, the TSST affects various subjective stress measures, as assessed using self-reports (e.g., Campbell & Ehlert, 2012; Rossi & Pourtois, 2012). The TSST begins with a brief preparation period during which the participants plan a speech that will be presented in front of a committee (5 minutes). The speech, they are informed, should convince the committee members that they are the perfect applicant for a desirable job. The selection panel members are introduced as trained in monitoring nonverbal behavior and the participants are informed that a video recording will be made of the speech, to be analyzed later by the committee members. Next, the participants deliver the speech (5 minutes) and perform an oral arithmetic task (5 minutes). The committee was composed of two experimenters that were not acquainted with the participants and were trained to provide no facial or verbal feedback to the participants. Two alterations of the original TSST were made in the current study: (a) The participants were informed that extra course credit will be given to those who will convince the committee to hire them. (b) The WM task replaced the mental arithmetic test, enabling the assessment of WM performance under stress (following Elzinga & Roelofs, 2005).
The current study also included a control condition, termed noStress (like Het, Rohleder, Schoofs, Kirschbaum, & Wolf, 2009; Wiemers, Schoofs, & Wolf, 2013). It preserves the cognitive and social components of the TSST, while reducing the stress component of the manipulation. More specifically, participants were notified that they were assigned to a control, no-stress, condition and that the committee members were already convinced that they were the “perfect applicants” for the job. During the preparation period (5 minutes) they reviewed their curriculum vitae (CV) and wrote points for discussion with the committee members. During the test period (5 minutes), the committee members introduced themselves to the participants and held a friendly, informal, conversation about their curriculum vitae (CV) The participants were informed that the conversation would not be recorded. Finally, the participants performed the WM task. Committee members stayed in the room during this time, but notified the participants that they were not observing their performance, and this was emphasized by visibly using their mobile phones.
Data analysis
Baseline comparisons (t1)
Baseline group differences were analyzed using ANOVAs and chi-square analyses for parametric and nonparametric variables, respectively.
Analyses of objective and subjective stress indicators
Subjective stress indicators were analyzed in the following manner: (a) To confirm stress induction, the STAI-S and VAS were analyzed using a 2 × 2 repeated-measures MANOVA, with stimulation (tDCS vs. shamStimulation) and stress manipulation (STRESS vs. noStress) as between-subjects factors, and time (baseline/follow-up) as a within-subjects factor. (b) To validate the subjective stress indicators, Pearson product-moment correlations were performed between the change in cortisol level and the change in self-reported stress (i.e., STAI-S and VAS). Regarding objective stress indicators, changes in cortisol level from the second to the third saliva test were assessed using independent-samples t-tests, performed separately for each tDCS stimulation group.
Analyses of WM performance
Accuracy (% correct responses) in the n-back task was analyzed using a 2 × 2 (stimulation × stress manipulation) ANOVA. Similar ANOVAs were performed for the n-back task’s reaction time and inverse efficiency score (IESFootnote 4). These analyses were repeated using performance in the CogState identification task as a covariate, with similar findings (not presented). In all analyses, independent-samples t tests were used to evaluate the direction of significant interactions. Finally, it was suggested by an anonymous reviewer that the duration of the n-back task (12.5 min) may have led to a temporal effect (i.e., stronger effects early in the task). Therefore, the analyses were repeated with the participants’ performance in each of the four rounds of the n-back task as the outcome measure. In these analyses, Bonferroni correction was employed to keep the total chance of erroneously reporting a difference below 5% (α set to .0125).
Results
Baseline comparisons (t1)
No significant group differences were found at baseline (i.e., trait anxiety, visual attention, cortisol level and rate of smoking/hormonal contraceptive use). See Table 1.
Analyses of objective and subjective stress indicators
Significant correlations were found, beyond all study groups, between change in cortisol level and change in STAI-S (r = .593, p < .001), as well as between cortisol level change and VAS change (r = .564, p < .001). The MANOVA of the subjective stress indicators revealed a significant Time × Stress-Manipulation interaction, STAI-S: F(1, 64) = 23.170, p < .001; VAS: F(1, 64) = 22.515, p < .001. There was a significantly larger subjective stress reaction in the STRESS condition than the noStress condition. This was evident for both the STAI-S and VAS and in both stimulation groups (tDCS/shamStimulation). In other words, the stress manipulation increased subjective stress, regardless of the stimulation condition. No other significant main effects or interactions were found. See Table 2 and Fig. 3.
As shown in Fig. 4, the noStress and STRESS groups significantly differed in t2–t3 cortisol level change in the shamStimulation condition, t(15) = −2.228, p = .042. In contrast, the group difference was not significant under tDCS, t(17) = −0.098, p = .923.
In summary, both subjective and objective stress indicators increased between t2 and t3 in the shamStimulation+STRESS group. In contrast, only the subjective indicators increased between t2 and t3 in the tDCS+STRESS group.
Analyses of WM performance
No significant stimulation or stress-manipulation main effects were found for the n-back accuracy measure, F(1, 65) = 0.074, p = .786; F(1, 65) = 0.017, p = .896, respectively. A significant Stimulation × Stress Manipulation interaction, however, was evident, F(1, 65) = 6.208, p = .015. Follow-up analyses indicated the following:
-
a.
Effects of tDCS in each stress condition: Follow-up t tests, performed for each stress condition, were not significant, tDCS+noStress vs. shamStimulation+noStress: t(35) = 1.731, p = .092; tDCS+STRESS vs. shamStimulation+STRESS: t(30) = −1.794, p = .083. See Fig. 5. Conventional significance testing, however, cannot determine whether nonsignificant outcomes really indicate that the manipulation had no effect (Kraemer, 2019). To compensate for this limitation, it is helpful to calculate a Bayes factor.Footnote 5 Thereby, the fit of the data under the null hypothesis and the alternative hypothesis is assessed. The comparisons were, therefore, repeated using Bayesian analysis (for a discussion regarding the use of this analysis in brain stimulation studies, see Biel & Friedrich, 2018). These analyses were performed using an online Bayes factor calculator (found at http://pcl.missouri.edu/bf-two-sample) and in accordance with Rouder, Speckman, Sun, Morey, and Iverson (2009). Prior probability distribution was based on Meiron and Lavidor (2013). For the noStress condition, the Bayes factor was in favor of H1 (scaled Jeffreys–Zellner–Siow [JZS] Bayes factor = 1.007, scaled-information Bayes factor = 1.408). More specifically, it suggested that in the noSTRESS conditions participants after tDCS had higher accuracy scores compared to those that underwent sham stimulation. Bayesian analysis suggested an opposite pattern (i.e., participants that underwent tDCS had lower accuracy scores compared to those that underwent sham stimulation) in the STRESS condition (scaled JZS Bayes factor = 1.114, scaled-information Bayes factor = 1.554). The analyses therefore supported the possibility that active tDCS enhanced WM performance in the noStress condition, while having an opposite effect in the STRESS condition. However, according to the interpretive guidelines of Lee and Wagenmakers (2013, p. 105) this support should be regarded as weak.
-
b.
Effects of stress in each tDCS condition: Stress significantly decreased performance in the tDCS groups (tDCS+STRESS vs. tDCS+noStress), t(33) = −2.285, p = .029. In contrast, it did not significantly affect WM performance in the sham groups (shamStimulation+STRESS vs. shamStimulation+noStress), t(32) = 1.383, p = .176. Interestingly, shamStimulation+STRESS participants were more accurate in the n-back task than the shamStimulation+noStress participants (i.e., those that were not subjected to the stress manipulation). However, the difference, as noted earlier, was not significant.
The analyses that were performed separately on each round of the n-back task were generally uninformative. More specifically, only the Stimulation × Stress Manipulation interaction for accuracy scores in the second round was significant, F(1, 65) = 7.615, p = .008. Follow-up t tests, performed separately for each stress condition, revealed that tDCS significantly impaired performance in the STRESS condition, t(30) = −2.310, p = .028. In contrast, the difference in the noSTRESS condition was not significant, t(35) = 1.524, p = .137. Follow-up t tests, performed separately for each stimulation condition, were not significant, though in the same direction as the analyses for the overall accuracy in the n-back task, shamStimulation+STRESS vs. shamStimulation+noStress: t(35) = 2.028, p = .051; tDCS+STRESS vs. tDCS+noStress: t(35) = −1.884, p = .068.
No significant effects were found for the n-back task’s reaction time measure, stimulation: F(1, 65) = 1.074, p = .304; stress manipulation: F(1, 65) = 0.457, p = .501; interaction: F(1, 65) = 0.116, p = .735), as well as IES, stimulation: F(1, 65) = 0.631, p = .430; stress manipulation: F(1, 65) = 0.152, p = .698; interaction: F(1, 65) = 0.301, p = .585.
Discussion
The current study focused on an often-neglected parameter, stress, by examining the possible interactive effect of tDCS and social stress on WM performance. This was accomplished by using a 2 × 2 research design in which stimulation (tDCS vs. shamStimulation) and stress manipulation (STRESS vs. noStress) were manipulated. This more elaborate research design builds upon earlier studies that manipulated only one of the factors (Antal et al., 2014; Meiron & Lavidor, 2013; Schoofs et al., 2008) and is similar to a more recent work that we were not aware of when designing the current study (Bogdanov & Schwabe, 2016). It is also in line with recent calls to delineate specific conditions and circumstances mediating tDCS effects (Hill et al., 2016; Horvath et al., 2014; Horvath, Forte, & Carter, 2015; Mancuso et al., 2016).
Our main finding indicated a significant disordinal interaction between dlPFC tDCS and stress manipulation. Follow-up analyses were not significant (i.e., p < .10). Considering the ongoing debate regarding the interpretation of “marginally significant” effects (Pritschet, Powell, & Horne, 2016) and taking into account the limitations of decisions based on conventional analyses (Kraemer, 2019; Wasserstein, 2016), we used Bayesian analysis to ascertain the relative support given to two hypotheses (H0 and H1) by the data. This contrasts with using a p value, which is calculated with reference to only the null hypothesis (Goodman, 2005). These analyses provided some support, though weak, for the possibility that active tDCS enhanced WM performance in the noStress condition, while having an opposite effect in the STRESS condition. This possibility should, however, be considered as speculative at this point and awaits further research. Another approach for conceptualizing this finding, awaiting further research, is suggested by Fig. 5. More specifically, applying one type of manipulation, either tDCS or stress (noStress+tDCS and STRESS+sham, respectively), seems to enhance WM performance compared with a control condition (noStress+sham). In contrast, simultaneously subjecting participants to both types of manipulations (tDCS+STRESS) seems to negate the enhancing effects of each manipulation, when performed separately. Such an interpretation is reminiscent of the inverted U hypothesis (see Arnsten, Raskind, Taylor, & Connor, 2015; Diamond, Campbell, Park, Halonen, & Zoladz, 2007; Goldman-Rakic, Muly, & Williams, 2000; Teigen, 1994). Perhaps inducing either moderate stress or applying dlPFC tDCS enhances executive attention and enables participants to reach an optimal zone of WM performance. In contrast, participants may reach a state of overload (i.e., impairing their WM performance) when subjected to overwhelming stress (Arnsten, 1998, 2009; Arnsten et al., 2015) or, as in the current study, when mild stress and tDCS are combined (tDCS+STRESS group). According to this conceptualization, right dlPFC tDCS and stress modulate overlapping PFC neuronal networks and, consequently, have deleterious effect when combined.
Caution is warranted with these inferences. While the Stimulation × Stress Manipulation interaction was significant, follow-up analyses of the main effects were not significant (i.e., p < .10), and Bayesian analyses provided only weak support for an interactive effect. Any inferences should therefore be considered as tentative and await further research. In addition, the suggestion that active tDCS may enhance WM performance when the participant is not subjected to stress is in line with previous findings. For example, Meiron and Lavidor (2013) found right dlPFC tDCS to improve the performance of females in a verbal n-back task. However, the suggestion that active tDCS impairs WM performance when performed in a stressful condition is somewhat surprising and therefore warrants further research. Positive effects of stress on various cognitive functions, including WM, were reported in the past (e.g., Duncko, Johnson, Merikangas, & Grillon, 2009; Weerda, Muehlhan, Wolf, & Thiel, 2010). Most studies, however, indicated a negative effect (e.g., Qin, Hermans, van Marle, Luo, & Fernández, 2009; Shields, Sazma, & Yonelinas, 2016). Regretfully, earlier studies are sparse and differ in methodology and design, challenging attempts to reach overarching conclusions. This is evident even when comparing the current study with that of Bogdanov and Schwabe (2016), a study that used a relatively similar design. For example, the mental arithmetic test, part of the TSST, was replaced by the WM task in the current study. This may explain the lower levels of subjective stress that were reported in the current study, compared with Bogdanov and Schwabe (2016), circa 40 and 65 in a 0–100 VAS, respectively. Moreover, the WM task in Bogdanov and Schwabe (2016) was performed under tDCS (online stimulation), while in the present study the task was performed about 15 minutes after stimulation ended (off-line stimulation). Clearly, attaining a unitary theoretical understanding of the interactive effects of dlPFC tDCS and stress will necessitate further research and accumulated data based on studies using similar methodologies.
Certain limitations of the study are important to note. First, there was a possible ceiling effect in the n-back task (86.93% ± 8.26% correct responses beyond study groups). This raises the possibility that a larger effect may have been demonstrated with a more challenging task (e.g., using higher WM loads, shorter stimuli durations, and/or shorter response-time windows). Relatedly, the length of the n-back task (12.5 min) may have obscured effects (i.e., tDCS/stress effects may have weakened with the passage of time). Our analyses found stronger effects in the second round out of four of the task, suggesting that this possibility is less likely. However, effects in the first round of the task may have been obscured due to the novelty of the task for the participants (i.e., larger variance in performance obscuring effects). More research is therefore needed to assess the temporal effects of both the tDCS the stress manipulations. Second, only females were analyzed in the current study. In light of the known gender differences in response to tDCS and stress (e.g., Campbell & Ehlert, 2012; Kudielka et al., 2007; Meiron & Lavidor, 2013; Schoofs et al., 2008), future investigations should evaluate possible gender effects on WM performance in stress-related conditions. Finally, there were between seven and 10 valid cortisol samples in each group (see Fig. 1). This limited the analyses that could be used in the current study (Pye, Taylor, Clay-Williams, & Braithwaite, 2016) and calls for larger samples, allowing more extensive analyses in future studies (e.g., differences between subgroups, changes over time). This should be part of a larger research endeavor aimed at clarifying the neural mechanisms through which tDCS and social stress interact. For example, an inverted U relationship between stress and PFC-dependent performance was noted in studies assessing the role of catecholamines (Arnsten, 1998, 2009; Arnsten et al., 2015).
Overall, the current study’s findings indicate that stress may be a key factor that interacts with dlPFC tDCS to affect WM performance. Hopefully, further research will uncover the mechanisms and pathways underlying these interactive effects on WM performance.
Notes
Although it is more common to place the anode over the left dlPFC when attempting to influence verbal WM, we selected a unilateral anodal right dlPFC montage. It was previously used by Meiron and Lavidor (2013), resulting in a significant improvement in WM during right dlPFC tDCS versus sham stimulation. In contrast, left dlPFC stimulation enhanced WM performance in males, while females showed a nonsignificant reduction in performance. In addition, fMRI studies (Perlstein, Carter, Noll, & Cohen, 2001; W. Schneider, 2003) support the importance of right dlPFC in behavioral regulation and verbal WM performance in both healthy participants and those with psychiatric disorders.
Prior to the actual task, the participant read the instructions and performed a practice stage that included seven trials. This allowed any misunderstandings to be clarified by the experimenter.
Note that tDCS is probably effective both when performed online or off-line (i.e., just before the WM task; see Oliveira et al., 2013). Our goal in the current study was to evaluate whether tDCS could prevent stress-induced WM impairment. We therefore chose an off-line stimulation design.
The IES was proposed to control for potential speed–accuracy trade-off and was used in previous studies (e.g., Adelhofer, Gohil, Passow, Beste, & Li, 2019; but see Bruyer & Brysbaert, 2011). This score is calculated by dividing mean RT of correct responses by the proportion of correct responses (Townsend & Ashby, 1978), is given in milliseconds and can be interpreted like mean reaction time (i.e., smaller IES indicates better performance in the n-back task).
Bayes factor represents the ratio between the probability of getting the result assuming the null hypothesis (H0) is true divided by the probability of getting the result assuming the alternative hypothesis (HA) is true (for a comprehensive review, see Goodman, 2005).
References
Adelhofer, N., Gohil, K., Passow, S., Beste, C., & Li, S. C. (2019). Lateral prefrontal anodal transcranial direct current stimulation augments resolution of auditory perceptual-attentional conflicts. NeuroImage, 199, 217–227. doi:https://doi.org/10.1016/j.neuroimage.2019.05.009
Allen, A. P., Kennedy, P. J., Cryan, J. F., Dinan, T. G., & Clarke, G. (2014). Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neuroscience & Biobehavioral Reviews, 38, 94–124. doi:https://doi.org/10.1016/j.neubiorev.2013.11.005
Antal, A., Fischer, T., Saiote, C., Miller, R., Chaieb, L., Wang, D. J. J.,… Kirschbaum, C. (2014). Transcranial electrical stimulation modifies the neuronal response to psychosocial stress exposure. Human Brain Mapping, 35(8), 3750–3759. doi:https://doi.org/10.1002/hbm.22434
Arnsten, A. F. (1998). The biology of being frazzled. Science, 280 (5370), 1711–1712. doi:https://doi.org/10.1126/science.280.5370.1711
Arnsten, A. F. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience, 10(6), 410–422. doi:https://doi.org/10.1038/nrn2648
Arnsten, A. F. (2015). Stress weakens prefrontal networks: Molecular insults to higher cognition. Nature Neuroscience, 18(10), 1376–1385. doi:https://doi.org/10.1038/nn.4087
Arnsten, A. F., Raskind, M. A., Taylor, F. B., & Connor, D. F. (2015). The effects of stress exposure on prefrontal cortex: Translating basic research into successful treatments for post-traumatic stress disorder. Neurobiology of Stress. doi:https://doi.org/10.1016/j.ynstr.2014.10.002
Bell, E. C., Willson, M. C., Wilman, A. H., Dave, S., & Silverstone, P. H. (2006). Males and females differ in brain activation during cognitive tasks. NeuroImage, 30(2), 529–538. doi:https://doi.org/10.1016/J.NEUROIMAGE.2005.09.049
Berryhill, M. E., Peterson, D. J., Jones, K. T., & Stephens, J. A. (2014). Hits and misses: Leveraging tDCS to advance cognitive research. Frontiers in Psychology, 5, 800. doi:https://doi.org/10.3389/fpsyg.2014.00800
Biel, A. L., & Friedrich, E. V. C. (2018). Why you should report Bayes factors in your transcranial brain stimulation studies. Frontiers in Psychology, 9, 1125. doi:https://doi.org/10.3389/fpsyg.2018.01125
Bogdanov, M., & Schwabe, L. (2016). Transcranial stimulation of the dorsolateral prefrontal cortex prevents stress-induced working memory deficits. Journal of Neuroscience, 36(4), 1429–1437. doi:https://doi.org/10.1523/JNEUROSCI.3687-15.2016
Bremner, J. D. (1999). Does stress damage the brain? Biological Psychiatry, 45(7), 797–805. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10202566
Brunoni, A. R., Nitsche, M. A., Bolognini, N., Bikson, M., Wagner, T., Merabet, L., … Bolognini, N. (2013). Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimulation, 5(3), 175–195. doi:https://doi.org/10.1016/j.brs.2011.03.002.Clinical
Brunoni, A. R., Vanderhasselt, M. A., Boggio, P. S., Fregni, F., Dantas, E. M., Mill, J. G., … Benseñor, I. M. (2012). Polarity- and valence-dependent effects of prefrontal transcranial direct current stimulation on heart rate variability and salivary cortisol. Psychoneuroendocrinology, 1–9. doi:https://doi.org/10.1016/j.psyneuen.2012.04.020
Bruyer, R., & Brysbaert, M. (2011). Combining speed and accuracy in cognitive psychology: Is the inverse efficiency score (IES) a better dependent variable than the mean reaction time (RT) and the percentage of errors (PE)?. Psychologica Belgica, 51(1), 5–13. doi:https://doi.org/10.5334/pb-51-1-5
Campbell, J., & Ehlert, U. (2012). Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37(8), 1111–1134. doi:https://doi.org/10.1016/j.psyneuen.2011.12.010
Coffman, B. A., Clark, V. P., & Parasuraman, R. (2014). Battery powered thought: Enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. NeuroImage, 85, 895–908. doi:https://doi.org/10.1016/j.neuroimage.2013.07.083
Diamond, D. M., Campbell, A. M., Park, C. R., Halonen, J., & Zoladz, P. R. (2007). The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plasticity, 2007, 1–33. doi:https://doi.org/10.1155/2007/60803
Dickerson, S. S., & Kemeny, M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. doi:https://doi.org/10.1037/0033-2909.130.3.355
Duncko, R., Johnson, L., Merikangas, K., & Grillon, C. (2009). Working memory performance after acute exposure to the cold pressor stress in healthy volunteers. Neurobiology of Learning and Memory, 91(4), 377–381. doi:https://doi.org/10.1016/j.nlm.2009.01.006
Elzinga, B. M., & Roelofs, K. (2005). Cortisol-induced impairments of working memory require acute sympathetic activation. Behavioral Neuroscience, 119(1), 98–103. doi:https://doi.org/10.1037/0735-7044.119.1.98
Fitzgerald, P. B., Maller, J. J., Hoy, K. E., Thomson, R., & Daskalakis, Z. J. (2009). Exploring the optimal site for the localization of dorsolateral prefrontal cortex in brain stimulation experiments. Brain Stimulation, 2(4), 234–237. doi:https://doi.org/10.1016/j.brs.2009.03.002
Folkman, S. (2013). Stress: Appraisal and coping. In M. D. Gellman & J. Rick Turner (Eds.), Encyclopedia of behavioral medicine (pp. 1913–1915). New York, NY: Springer. doi:https://doi.org/10.1007/978-1-4419-1005-9_215
Fregni, F., Boggio, P. S., Nitsche, M. A., Rigonatti, S. P., & Pascual-Leone, A. (2006). Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depression and Anxiety, 23(8), 482–484. doi:https://doi.org/10.1002/da.20201
Frisch, J. U., Häusser, J. A., & Mojzisch, A. (2015). The Trier Social Stress Test as a paradigm to study how people respond to threat in social interactions. Frontiers in Psychology, 6, 14. doi:https://doi.org/10.3389/fpsyg.2015.00014
Gaab, J., Rohleder, N., Nater, U. M., & Ehlert, U. (2005). Psychological determinants of the cortisol stress response: The role of anticipatory cognitive appraisal. Psychoneuroendocrinology, 30(6), 599–610. doi:https://doi.org/10.1016/j.psyneuen.2005.02.001
Goldman-Rakic, P. S., Muly, E. C., & Williams, G. (2000). D1 receptors in prefrontal cells and circuits. Brain Research Reviews, 31(2/3), 295–301. doi:https://doi.org/10.1016/S0165-0173(99)00045-4
Goldstein, J. M., Jerram, M., Poldrack, R., Anagnoson, R., Breiter, H. C., Makris, N.,… Seidman, L. J. (2005). Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology, 19(4), 509–519. doi:https://doi.org/10.1037/0894-4105.19.4.509
Goodman, S. N. (2005). Introduction to Bayesian methods I: Measuring the strength of evidence. Clinical Trials, 2, 282–290; discussion 301–284, 364–278. doi:https://doi.org/10.1191/1740774505cn098oa
Het, S., Rohleder, N., Schoofs, D., Kirschbaum, C., & Wolf, O. T. (2009). Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test.’ Psychoneuroendocrinology, 34(7), 1075–1086. doi:https://doi.org/10.1016/j.psyneuen.2009.02.008
Hill, A. T., Fitzgerald, P. B., & Hoy, K. E. (2016). Effects of anodal transcranial direct current stimulation on working memory: A systematic review and meta-analysis of findings from healthy and neuropsychiatric populations. Brain Stimulation, 9(2), 197–208. doi:https://doi.org/10.1016/j.brs.2015.10.006
Horvath, J. C., Carter, O., & Forte, J. D. (2014). Transcranial direct current stimulation: Five important issues we aren’t discussing (but probably should be). Frontiers in Systems Neuroscience, 8. doi:https://doi.org/10.3389/fnsys.2014.00002
Horvath, J. C., Forte, J. D., & Carter, O. (2015). Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS). Brain Stimulation, 8(3), 535–550. doi:https://doi.org/10.1016/j.brs.2015.01.400
Jaeggi, S. M., Studer-Luethi, B., Buschkuehl, M., Su, Y. F., Jonides, J., & Perrig, W. J. (2010). The relationship between n-back performance and matrix reasoning—implications for training and transfer. Intelligence, 38(6), 625–635. doi:https://doi.org/10.1016/j.intell.2010.09.001
Kane, M. J., & Engle, R. W. (2002). The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review, 9(4), 637–671. doi:https://doi.org/10.3758/BF03196323
Kirchner, W. K. (1958). Age differences in short-term retention of rapidly changing information. Journal of Experimental Psychology, 55(4), 352–358. doi:https://doi.org/10.1037/h0043688
Kirschbaum, C., Pirke, K. M., & Hellhammer, D. H. (1993). The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1/2), 76–81. doi:https://doi.org/10.1159/000119004
Kraemer, H. C. (2019). Is it time to ban the p value?. JAMA Psychiatry.https://doi.org/10.1001/jamapsychiatry.2019.1965
Kudielka, B. M., Hellhammer, H., & Kirschbaum, C. (2007). Ten years of research with the Trier Social Stress Test. Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior, 56, 83.
LaBar, K. S., & Cabeza, R. (2006). Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience, 7(1), 54–64. doi:https://doi.org/10.1038/nrn1825
Lapenta, O., Valasek, C., Brunoni, A., & Boggio, P. (2014). An ethical discussion of the use of transcranial direct current stimulation for cognitive enhancement in healthy individuals: A fictional case study. Psychology & Neuroscience, 7(2), 175–180. doi:https://doi.org/10.3922/j.psns.2014.010
Lazarus, R. S., & Folkman, S. (1984). Stress, appraisal, and coping. New York, NY: Springer. Retrieved from https://books.google.com/books?id=i-ySQQuUpr8C&pgis=1
Lee, M. D., & Wagenmakers, E. J. (2013). Bayesian cognitive modeling: A practical course. Cambridge, UK: Cambridge University Press.doi:https://doi.org/10.1017/CBO9781139087759
Lim, Y. Y., Jaeger, J., Harrington, K., Ashwood, T., Ellis, K. A., Stoffler, A.,… Maruff, P. (2013). Three-month stability of the CogState Brief Battery in healthy older adults, mild cognitive impairment, and Alzheimer’s disease: Results from the Australian Imaging, Biomarkers, and Lifestyle-Rate of Change Substudy (AIBL-ROCS). Archives of Clinical Neuropsychology, 28(4), 320–330. doi:https://doi.org/10.1093/arclin/act021
Mancuso, L. E., Ilieva, I. P., Hamilton, R. H., & Farah, M. J. (2016). Does transcranial direct current stimulation improve healthy working memory?: A meta-analytic review. Journal of Cognitive Neuroscience, 28(8), 1063–1089. doi:https://doi.org/10.1162/jocn_a_00956
Meiron, O., & Lavidor, M. (2013). Unilateral prefrontal direct current stimulation effects are modulated by working memory load and gender. Brain Stimulation, 6(3), 440–447. doi:https://doi.org/10.1016/j.brs.2012.05.014
Meiron, O., & Lavidor, M. (2014). Prefrontal oscillatory stimulation modulates access to cognitive control references in retrospective metacognitive commentary. Clinical Neurophysiology, 125, 77–82. doi:https://doi.org/10.1016/j.clinph.2013.06.013
Nitsche, M. A., Cohen, L., Wassermann, E. M., Priori, A., Lang, N., Antal, A., . . . Pascual-Leone, A. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1(3), 206–223. doi:https://doi.org/10.1016/j.brs.2008.06.004
Oliveira, J. F., Zanão, T. A., Valiengo, L., Lotufo, P. A., Benseñor, I. M., Fregni, F., & Brunoni, A. R. (2013). Acute working memory improvement after tDCS in antidepressant-free patients with major depressive disorder. Neuroscience letters, 537, 60–64.
Perlstein, W. M., Carter, C. S., Noll, D. C., & Cohen, J. D. (2001). Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. American Journal of Psychiatry, 158(7), 1105–1113. doi:https://doi.org/10.1176/appi.ajp.158.7.1105
Priori, A., Berardelli, A., Rona, S., Accornero, N., & Manfredi, M. (1998). Polarization of the human motor cortex through the scalp. NeuroReport, 9(10), 2257–2260.
Pritschet, L., Powell, D., & Horne, Z. (2016). Marginally significant effects as evidence for hypotheses: Changing attitudes over four decades. Psychological Science, 27, 1036–1042. doi:https://doi.org/10.1177/0956797616645672
Pye, V., Taylor, N., Clay-Williams, R., & Braithwaite, J. (2016). When is enough, enough? Understanding and solving your sample size problems in health services research. BMC Research Notes 9, 90. doi:https://doi.org/10.1186/s13104-016-1893-x
Qin, S., Hermans, E. J., van Marle, H. J. F., Luo, J., & Fernández, G. (2009). Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biological Psychiatry, 66(1), 25–32. doi:https://doi.org/10.1016/j.biopsych.2009.03.006
Rossi, V., & Pourtois, G. (2012). Transient state-dependent fluctuations in anxiety measured using STAI, POMS, PANAS or VAS: A comparative review. Anxiety, Stress, and Coping, 25(6), 603–645. doi:https://doi.org/10.1080/10615806.2011.582948
Rouder, J. N., Speckman, P. L., Sun, D., Morey, R. D., & Iverson, G. (2009). Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review, 16, 225–237. doi:https://doi.org/10.3758/PBR.16.2.225
Schmiedek, F., Oberauer, K., Wilhelm, O., Suss, H. M., & Wittmann, W. W. (2007). Individual differences in components of reaction time distributions and their relations to working memory and intelligence. Journal of Experimental Psychology: General, 136, 414–429. doi:https://doi.org/10.1037/0096-3445.136.3.414
Schneider, M., Walter, H., Moessnang, C., Schäfer, A., Erk, S., Mohnke, S.,… Tost, H. (2017). Altered DLPFC–hippocampus connectivity during working memory: Independent replication and disorder specificity of a putative genetic risk phenotype for schizophrenia. Schizophrenia Bulletin, 43(5), 1114–1122. doi:https://doi.org/10.1093/schbul/sbx001
Schneider, W. (2003). Controlled & automatic processing: Behavior, theory, and biological mechanisms. Cognitive Science, 27(3), 525–559. doi:https://doi.org/10.1016/S0364-0213(03)00011-9
Schoofs, D., Preuß, D., & Wolf, O. T. (2008). Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology, 33(5), 643–653. doi:https://doi.org/10.1016/j.psyneuen.2008.02.004
Schutter, D. J. L. G., & Wischnewski, M. (2016). A meta-analytic study of exogenous oscillatory electric potentials in neuroenhancement. Neuropsychologia, 86, 110–118. doi:https://doi.org/10.1016/j.neuropsychologia.2016.04.011
Shields, G. S., Sazma, M. A., & Yonelinas, A. P. (2016). The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neuroscience and Biobehavioral Reviews, 68, 651–668. doi:https://doi.org/10.1016/j.neubiorev.2016.06.038
Spielberger, C. D., Gorsuch, R. L., & Lushene, R. E. (1970). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. Retrieved from http://ubir.buffalo.edu/xmlui/handle/10477/2895
Teigen, K. H. (1994). Yerkes-Dodson: A law for all seasons. Theory & Psychology, 4(4), 525–547. doi:https://doi.org/10.1177/0959354394044004
Townsend, J. T., & Ashby, F. G. (1978). Methods of modeling capacity in simple processing systems. In N. J. Castellan, Jr., & F. Restle (Eds.), Cognitive theory (Vol. 3, pp. 211–252). Psychology Press. doi:https://doi.org/10.4324/9781315802473-14
Utz, K. S., Dimova, V., Oppenländer, K., & Kerkhoff, G. (2010). Electrified minds: Transcranial direct current stimulation (tDCS) and Galvanic Vestibular Stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology—A review of current data and future implications. Neuropsychologia, 48(10), 2789–2810. doi:https://doi.org/10.1016/j.neuropsychologia.2010.06.002
Wasserstein, R., (2016). The ASA statement on p-values: Context, process, and purpose. The American Statistician, 70(2), 129–133. doi:https://doi.org/10.1080/00031305.2016.1154108
Weerda, R., Muehlhan, M., Wolf, O. T., & Thiel, C. M. (2010). Effects of acute psychosocial stress on working memory related brain activity in men. Human Brain Mapping, 31(9), 1418–1429. doi:https://doi.org/10.1002/hbm.20945
Wiemers, U. S., Schoofs, D., & Wolf, O. T. (2013). A friendly version of the Trier Social Stress Test does not activate the HPA axis in healthy men and women. Stress, 16(2), 254–260. doi:https://doi.org/10.3109/10253890.2012.714427
Wolf, O. T. (2006). Effects of stress hormones on the structure and function of the human brain. Expert Review of Endocrinology and Metabolism, 1(5), 10. doi:https://doi.org/10.1586/17446651.1.5.623
Zaehle, T., Sandmann, P., Thorne, J. D., Jäncke, L., & Herrmann, C. S. (2011). Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: Combined behavioural and electrophysiological evidence. BMC Neuroscience, 12(1), 2. doi:https://doi.org/10.1186/1471-2202-12-2
Acknowledgements
We would like to thank the research assistants at the Laboratory of Forensic and Clinical Neuropsychology, Ariel University: Lior Golan, Jonathan David, Noy Cohen, Astar Lev, Sees Fromm and Liat Maor.
Funding
This work was funded by the IDF Medical Forces (Grant No. 4440693179).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest in the conduct and reporting of this research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Analyses of participants’ working memory performance revealed a significant interaction between the two manipulations (tDCS and social stress).
• Conventional follow-up analyses were not significant (i.e., ps=.083 / .093). Bayesian analyses, however, provided initial—though weak—support for the possibility that tDCS has opposing effects on working memory, depending on the participants’ stress level (i.e., enhancing working memory at lower levels of stress, while decreasing performance when participants are subjected to social stress). Further research, however, is needed before firm conclusions can be reached.
• It is recommended to consider the effects of stress when studying noninvasive brain stimulation.
Rights and permissions
About this article
Cite this article
Ankri, Y.L., Braw, Y., Luboshits, G. et al. The effects of stress and transcranial direct current stimulation (tDCS) on working memory: A randomized controlled trial. Cogn Affect Behav Neurosci 20, 103–114 (2020). https://doi.org/10.3758/s13415-019-00755-7
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-019-00755-7