Abstract
Core samples to a depth of approximately 50 m and undisturbed soil samples were retrieved from eight locations in the Saga Plain, Japan, to investigate the effects of the depositional environment and post-depositional processes on the engineering properties of Quaternary clays in the Saga Plain. The engineering properties of these soil samples were measured, and the test results showed that the clays in the Saga Plain have a high void ratio, high water content, high compressibility, high sensitivity, and low stiffness. The one-dimensional compression curves plotted above the sedimentation compression line proposed by Burland, and the compression index was high, which indicated that the clays had been cemented to some degree. The clays have high strength sensitivity and high stress sensitivity, indicating that they have strong structures. It was considered that a considerable amount of volcanic ash from the four large volcanic eruptions that occurred during the deposition of these clays settled into the clays, resulting in a certain degree of cementation, which was attributed as the main driver of the strong structure in these clays. Comparisons of the NaCl concentration, pH, and oxidation-reduction potential (ORP) of the clay samples representing the initial depositional environment and the current ground environment showed that salt leaching occurred in the Ariake clay formation, and that the ground environment changed. When the NaCl concentration was less than approximately 2 g/L, the strength sensitivity of the Ariake clay samples with low-activity clay minerals significantly increased due to the decrease in the liquid limit and subsequent significant decrease in the remolded undrained shear strength. Therefore, salt leaching was probably a main cause of the high strength sensitivity of the clays in the Saga Plain. The depositional environment at some locations changed from an alkaline, reducing environment, to an acidic, oxidative environment, with the potential to change into a strongly acidic environment, which could have deteriorated the strength of the cement/lime-treated clays in the Saga Plain.
Similar content being viewed by others
Introduction
The Saga Plain is located on the island of Kyushu, Japan, and is surrounded by the Sefuri Mountains, Kishima Mountains, Chikuhi Mountains, and Ariake Sea. The Saga Plain is a coastal lowland with an average elevation of approximately 2 m, and it is below sea level during high tide (Zhou et al. 2003). The groundwater in the Saga Plain is composed of shallow unconfined water with a water level of approximately 0.5 m below the ground surface and deeper confined water. The Quaternary deposits in the Saga Plain contain a 10- to 30-m-thick Holocene clay deposit and a Pleistocene deposit of alternating layers of clay, sand, and gravel. The expressway embankment and the foundations of some low buildings overlay the Holocene clay deposit, so the engineering properties of the Holocene clay need to be well-understood. Although the pile foundations of bridges and some high buildings overlay the Pleistocene sand or gravel deposit, the engineering properties of the Pleistocene clay just below the bearing stratum of the sand or gravel deposit must also be well-understood. Therefore, it is important to investigate and understand the engineering properties of the Holocene and Pleistocene clays in the Saga Plain.
Depositional environment significantly affects the macrostructure, microstructure, and engineering properties of clays. Chung and Finno (1992) investigated the effects of the depositional environment on the index properties, stress history, and shear strength of Chicago glacial clays and indicated that the depositional environment had a major impact on the engineering properties of the Chicago clays. Cecconi et al. (2010) reported that the engineering properties of the pyroclastic flow deposits of the Colli Albani volcanic complex were related to the geological origin of the deposits and their formation environment. Liu et al. (2011) presented the mineralogy and geotechnical properties of marine clays from Lianyungang, China, and discussed the correlations of their depositional history, depositional environmental, and geotechnical properties using statistical analysis. Wu et al. (2015) investigated the physical, mineralogical, and mechanical properties of Shanghai clays by performing laboratory tests, and they studied the depositional environment of the Shanghai clays, comparing it with those of the Ariake clay in Japan and Bangkok clay in Thailand. The effects of the depositional environment on the grain size distribution, clay mineralogy, and soil structure of the Shanghai clays were analyzed. Ye et al. (2018) indicated that the engineering properties of the marine clays were closely related to their depositional environments, and they investigated the effect of the Quaternary transgression and regression on the engineering properties of the Shanghai soft clays.
Post-depositional processes such as salt leaching and chemical weathering alter the chemistry of clays and thus influence the engineering properties of clays (Quigley 1980; Brand and Brenner 1981; Nguyen et al. 2013). The effect of salt leaching on the engineering properties of various marine clays has been investigated. Some researchers indicated that salt leaching resulted in a decreased liquid limit and remolded shear strength, and an increased compressibility and sensitivity (Bjerrum 1967; Moore et al. 1977; Woo and Moh 1977; Ismael 1993; Kim and Do 2011). However, other researchers reported that salt leaching resulted in an increased liquid limit and remolded shear strength (Warkentin and Yong 1962; Sridharan and Prakash 1999; He et al. 2014). Chemical weathering can transform one mineral into another or completely dissolve minerals (Mitchell and Soga 2005). Hino et al. (2012) reported that weathering resulted in the oxidation of pyrites and the generation of iron oxide, which influenced the engineering properties of the Holocene clay in the Saga Plain.
The engineering properties of clay deposits at a site are influenced by their complete history. Graham and Shields (1985) indicated that the engineering properties of glaciolacustrine clays arose from both of their depositional and post-depositional environments. Howayek et al. (2017) indicated that the geologic origin, depositional environment, and post-depositional environment play a critical role in determining both the composition and the structure of natural soils. Therefore, the study of the effect of depositional and post-depositional environments on the engineering properties of Quaternary clays can be of great assistance in understanding and accurately determining engineering properties in geotechnical practice. In recent years, a large amount of research has been carried out to investigate the depositional environment and engineering properties of the Quaternary clay deposits in coastal areas (Arulrajah et al. 2005; Horpibulsuk et al. 2007, 2011; Xu et al. 2009; Du et al. 2014; Shen et al. 2014).
In this study, the engineering properties of the Quaternary clays in the Saga Plain were investigated using core samples and undisturbed soil samples from approximately 50-m deep to the ground surface level at eight locations in the Saga Plain. Laboratory physical index testing, one-dimensional consolidation testing, unconfined compression testing, and laboratory vane shear testing were conducted to investigate the physical properties, compressibility, and undrained shear strength (su) of the clay samples. The depositional environment and geological setting of the Quaternary deposits in the Saga Plain were investigated by utilizing the obtained core samples along with the information from previous publications, and the effect of the depositional environment on the engineering properties of the clays was discussed. The changes in the post-depositional environment of the clays were investigated by comparing the NaCl concentration, pH, and oxidation-reduction potential (ORP) of the soil samples from the Saga Plain, representing the current ground environment, with those of soil samples from the seabed of the Ariake Sea, representing the initial depositional environment. The effect of the post-depositional processes, i.e., salt leaching and ground environment changes, on the engineering properties of the clays was also discussed.
Engineering properties of the Quaternary clays in the Saga Plain
Investigation locations and methods
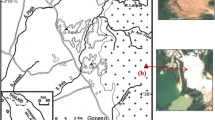
The investigation locations for this study are shown in Fig. 1. Boreholes were drilled and core samples were obtained at eight locations (A-1, A-2, A-3, A-4, A-5, A-6, A-7, and A-8) in the Saga Plain, and undisturbed soil samples were also retrieved using a Japanese standard thin-walled sampler. The extracted core samples were immediately enclosed in a core pack and then stored at a constant temperature of 20 °C in the laboratory to prevent weathering. The core samples were halved using a wire to prepare them for observation and photography. Next, the lithofacies, sedimentary facies, and colors were observed and recorded. The ORP, pH, and salt concentration were then promptly measured. A series of laboratory physical index tests, one-dimensional consolidation tests, unconfined compression tests, and laboratory vane shear tests were conducted on the undisturbed soil samples to obtain the physical and mechanical properties of the Quaternary clays in the Saga Plain.
The depositional environment of the Quaternary clays in the Saga Plain was investigated by utilizing the core samples and information from previous publications. The stratigraphic columns of the core samples from all eight locations are shown in Fig. 2. The Quaternary deposits consist of the Hasuike formation, Ariake clay formation, Mitagawa formation, Aso-4 pyroclastic flow deposit, Nakabaru formation, Takagise formation, Aso-3 pyroclastic flow deposit, and the Kawazoe formation. The details of the depositional environment and the geological setting of the Quaternary deposits in the Saga Plain are described in the “Effect of the depositional environment on the engineering properties” section.
At the seabed of the Ariake Sea, undisturbed soil samples with a height of 1 m were also obtained with a thin-walled sampler at the eight investigation locations (Y-1, Y-2, Y-3, Y-4, Y-5, Y-6, Y-7, and Y-8). The ORP, pH, and salt concentrations of these soil samples were measured and compared with those of soil samples from the Saga Plain to examine the changes in the post-depositional environment of the Quaternary clays in the Saga Plain.
Physical properties
The soil strata and their physical properties at two typical locations, A-2 and A-6, are shown in Fig. 3a and b, respectively. In Fig. 3, HU stands for the upper member of the Hasuike formation, HL stands for the lower member of the Hasuike formation, AC stands for the Ariake clay formation, M stands for the Mitagawa formation, Aso-4 stands for the Aso-4 pyroclastic flow deposit, Aso-3 stands for the Aso-3 pyroclastic flow deposit, NU stands for the upper member of the Nakabaru formation, NL stands for the lower member of the Nakabaru formation, TA stands for the Takagise formation, wp stands for the plastic limit, wL stands for the liquid limit, and wn stands for the natural water content.
The clay contents (< 5 μm) of the samples were in the range of 35–70%. X-ray diffraction patterns of the clay deposits in the Saga Plain showed that the main clay minerals were smectite, illite, vermiculite, chlorite, kaolinite, and halloysite, and that the predominant clay mineral was smectite, an in situ product from the pyroclastic material (Ohtsubo et al. 1995; Mizota and Longstaffe 1996). The plasticity indexes were generally in the range of 40–70%. The water contents in the Ariake clay formation were generally higher than 90% and higher than their liquid limits. The water contents in the Nakabaru and Takagise formations were generally less than 95% and less than their liquid limits. The measured void ratios were in the range of 1.8–4.2. The unit weight of the samples was approximately 15 kN/m3. Ohtsubo et al. (1995) analyzed the pore water chemistry of clay deposits in the Saga Plain using the extracted solution with a solid concentration of 10% by weight adjusted by distilled water, and the analysis results showed that for soils 0–20 m below the ground surface, the concentration of Na+ ranged from 0.03 to 0.20 N with the maximum value at 4 m, the concentrations of Mg2+ and Ca2+ ranged from 0.01 to 0.1 N, and the concentration of K+ was less than 0.01 N throughout the profile. Additionally, the concentration of Cl- ranged from 0.01 to 0.28 N, and the concentration of SO42- ranged from 0.1 to 0.35 N.
Compressibility properties
An understanding of compression behavior is important in geological and geotechnical engineering practice (Liu et al. 2013). The intrinsic compression line (ICL) is defined as the one-dimensional consolidation line of reconstituted soil with an initial water content between wL and 1.5wL. Burland (1990) reported that the ICL can be produced by the following equation:
where σ′v is the effective vertical stress. Additionally, Iv is the void index, and it can be calculated by the following equation:
where e is the void ratio, \( {e}_{100}^{\ast } \) is the void ratio corresponding to \( {\sigma}_{\mathrm{v}}^{\prime }=100\ \mathrm{kPa} \), and \( {C}_{\mathrm{c}}^{\ast } \) is the intrinsic compression index.
It has been further suggested that \( {e}_{100}^{\ast } \) and \( {C}_{\mathrm{c}}^{\ast } \) can be estimated from the void ratio at the liquid limit (eL) by the following equations:
Based on the measured data of several clays, Burland (1990) also introduced the sedimentation compression line (SCL), which plots the in situ void index (Iv0) against the in situ overburden stress (\( \log {\sigma}_{\mathrm{v}0}^{\prime } \)). The SCL has been used as a reference for investigating the structure of natural clays.
Conventional incremental loading (IL) consolidation tests were conducted to investigate the compressibility of the Quaternary clays in the Saga Plain. The one-dimensional compression curves for \( {I}_{\mathrm{v}}-\log {\sigma}_{\mathrm{v}}^{\prime } \) are shown for samples at the typical locations, A-2 and A-8, in Fig. 4a and b, respectively. Iv can be calculated with Eqs. (2)–(4), in which eL is calculated by the following equation:
where wL is the liquid limit, Gs is the specific gravity, and Sr is the degree of saturation.
The ICL and the SCL were also plotted as shown in the figures. For nearly all of the samples, the one-dimensional compression curves plotted above the SCL. With an increase in the vertical compression pressure, the curves gradually approached the SCL. Hong and Tsuchida (1999) indicated that the compression curves of most natural Ariake clays lay above the SCL due to cementation. Hong (2006) reported several one-dimensional compression curves for clays in the Saga Plain; the results were similar to the observations reported in this study. Pineda et al. (2016) reported that compression curves of natural clays initially plotted above the SCL and then approached the SCL and even merged with the ICL. For comparison, the one-dimensional compression curve for the sample at location Y-1 is shown in Fig. 4c. The one-dimensional compression curve plotted between the ICL and SCL for the clay in the seabed of Isahaya Bay. The test results in Fig. 4 indicate that the Quaternary clays in the Saga Plain might have undergone some degree of cementation and that they have strong structures.
The values of the compression index (Cc) of all the tested soil samples are shown in Fig. 5a (A-1, A-2, A-4 and A-6) and Fig. 5b (A-3, A-5, A-7 and A-8). The values of Cc were between 0.4 and 2.8, and most values ranged from 1 to 2. For the soils more than 20 m below the ground surface, as shown in Fig. 5a, the values of Cc were still quite high. In the Saga Plain, in many geotechnical engineering designs, the selected bearing layers for the pile foundations of some buildings and bridges are approximately 20-m deep. If the applied load is too large and the additional stress in the clay layer exceeds its yield stress, a large settlement may occur due to the compression of the clay layer below the sand-bearing layer. If this is the case, the designers should ensure that the yield stress of the clay layer below the sand-bearing layer is not exceeded.
Strength properties
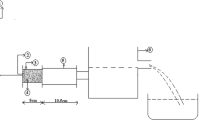
Figure 6a and b shows the variations in su with depth for the undisturbed specimens and the remolded specimens, and the strength sensitivity at two typical locations, A-2 and A-6. The strength sensitivity variations were determined by the ratio of su in the undisturbed state to that in the remolded state. Unconfined compression tests were conducted to measure su of the undisturbed specimens, and the undisturbed su was assumed to be half of the unconfined compression strength (qu). Laboratory vane shear tests were conducted to measure su of the remolded specimens. The soil sample was remolded and placed in a soil specimen container that was 16 mm in height and 10 mm in diameter. Then, the vane with a height of 4 cm and a diameter of 2 cm was pushed into the specimen and rotated at a rate of 6°/min until the peak strength was reached. Generally, the undisturbed and remolded su values both increased with depth. The minimum value of the undisturbed su was approximately 8 kPa, which was measured from the specimen collected approximately 3.4 m below the ground surface at location A-6. Miura et al. (1998) reported that the minimum value of su of the undisturbed samples from the Saga airport site was approximately 9 kPa, which is comparable with the results of this study. The values of the strength sensitivity ranged from 6 to 30. For comparison, the remolded and undisturbed su and the strength sensitivity for the sample at location Y-1 are shown in Fig. 6c. The values of the strength sensitivity are high, even for the young sediment at the seabed of Isahaya Bay.
Chandler (2000) defined the stress sensitivity as the ratio of the yield stress to the corresponding stress on the ICL at the same void index. The stress sensitivities of the tested samples were calculated. The relationship between the stress sensitivity and the strength sensitivity is shown in Fig. 7. The stress sensitivity tended to increase with the strength sensitivity. Therefore, the stress sensitivity could also be used as an index to investigate the structure of natural clays. Higher values of both the stress sensitivity and the strength sensitivity indicated that the Quaternary clays in the Saga Plain have strong structures.
The variations in the secant modulus that corresponded to a compressive stress of 0.5qu (E50) and the ratio of E50 to the undisturbed su (E50/su) with depth for two typical locations, A-2 and A-6, are depicted in Fig. 8a and b. Generally, the values of E50 increased with depth. The values of E50/su ranged from 100 to 260 for the soil samples at location A-2, and the values of E50/su ranged from 70 to 200 for the soil samples at location A-6. Therefore, the stiffness of the Quaternary clays in the Saga Plain is low.
Microscopic properties
Figure 9a and b shows vertical plane and horizontal plane scanning electron microscopy (SEM) images, respectively, of a sample from A-2 from the depth range of 7–8 m. The microstructure is composed of the aggregations of soil particles containing diatomite, the linkage of the aggregations, and the interaggregate and intraaggregate pores. More faces of platy particles can be observed on the horizontal plane, and more edges of platy particles can be observed on the vertical plane, which indicates the existence of a certain anisotropic microstructure caused by the overburden pressure. For comparison, vertical plane and horizontal plane SEM images of a sample from Y-1 from the depth range of 0–1 m are shown in Fig. 9c and d, respectively. There is no obvious difference between the images of the vertical and horizontal planes. The pore sizes are obviously larger, and the interaggregate bonds are weaker in the Y-1 sample compared with the A-2 sample. Therefore, the macroscopic properties of clays in the Saga Plain are controlled by the microscopic properties.
Effect of the depositional environment on the engineering properties
The Saga Plain is in the northern coast of the Ariake Sea. The Ariake Sea is a typical semi-closed inland bay, and it has an area of approximately 1700 km2, a length of 100 km, an average width of 18 km, and an average water depth of 20 m. The Quaternary period is divided into two epochs: the Pleistocene epoch, from 2.588 million years ago to 11.7 thousand years ago, and the Holocene epoch, from 11.7 thousand years ago to today (Cohen et al. 2013). During the Holocene transgression approximately 15,000 to 6000 years ago, the depositional environment at the northern coast of the Ariake Sea changed from a freshwater environment to a marine environment. The northern coastlines of the Ariake Sea during the three periods, approximately 7000 years ago, approximately 1800 years ago, and approximately 300 years ago, are shown in Fig. 10. In the past 7000 years, most of the Saga Plain has had a marine depositional environment. The clays deposited in this marine environment make up the Ariake clay formation. Similarly, the Takagise formation was deposited in a marine environment during the late Pleistocene transgression.
Changes in the northern coastline of the Ariake Sea (modified from Hino et al. 2014)
The Quaternary strata deposited in the Saga Plain are shown in Fig. 11. The Holocene deposits consist of the Ariake clay formation and Hasuike formation, which is divided into a lower member and an upper member. The late Pleistocene deposits include the Mitagawa formation, the Nakabaru formation, which is divided into a lower member and an upper member, and the Takagise formation. The Mitagawa and Nakabaru formations consist of alternating layers of gravel, clay, and sand, while the Takagise formation is a clay layer. The middle Pleistocene deposits are the Tateishi formation, the Kawazoe formation, and the Ushiya formation. The Tateishi formation is a gravel layer, while the Kawazoe and Ushiya formations consist of alternating layers of gravel, sand, and clay (Shimoyama et al. 2010).
Geological setting of the Saga Plain (modified from Shimoyama et al. 2010)
The Quaternary deposits in the Saga Plain contain volcanic ashes. During the Quaternary Period, four large volcanic eruptions occurred around the Saga Plain—the Kikai eruption and Aira eruption during the Holocene epoch, and the Aso-4 eruption (fourth largest eruption of the Aso volcano) and Aso-3 eruption (third largest eruption of the Aso volcano) during the Pleistocene epoch. As shown in Fig. 10, the Kikai-akahoya and Aira-Tn tephra layers are attributed to the Kikai and Aira eruptions, respectively. Similarly, the Aso-4 and Aso-3 pyroclastic flow deposits are attributed to the Aso-4 and Aso-3 eruptions, respectively.
The Quaternary clay deposits in the Saga Plain are deposits of fine soil particles eroded and transported from the watershed, which was widely covered with pyroclastic materials. Therefore, considerable amounts of volcanic ash might have been deposited with the clay deposits because the origin materials of the clays were the surrounding eroded soils, which contained volcanic ashes. Torrance and Ohtsubo (1995) reported that the silt and fine-sand fractions contained quartz, feldspars, cristobalite, and volcanic ash, and more than 20–30% of the silt and fine-sand fractions was volcanic ash, which was eroded from the upland sites along with the other sediments. Volcanic ashes around the Saga Plain contain a relatively high amount of cementation materials, such as calcium oxide (CaO) and magnesium oxide (MgO) (Matsui et al. 1963). The existence of volcanic ashes in the clay deposits has an important effect on their engineering properties; although quantifying the effect of pyroclastic ash on the compressibility and strength properties of clays by analyzing the variations in soil properties with depth is challenging, because the compressibility and strength properties of clays are related to their consolidation pressures, which increase with increasing depth, and it is difficult to quantify the amount of pyroclastic ash in the clay deposits. The deposition of volcanic ash within the clay deposits has resulted in a certain degree of cementation in the clays, and it is the main driver of the strong structure of the Quaternary clays in the Saga Plain.
Effect of the post-depositional processes on the engineering properties
Salt concentration is a measure of the salt content dissolved in water. pH is a measure of the acidity or alkalinity of a solution. ORP is a measure of the tendency of a substance to oxidize or reduce another substance. The salt concentration, pH, and ORP of the pore water in the soil samples, which are described as geoenvironmental indexes, can be used to identify the depositional and post-depositional environments of the clay deposits. In this study, the NaCl concentrations of the soil samples were measured, because the salt in the seawater was predominantly made of sodium (Na+) and chloride (Cl−) ions. The NaCl concentration, pH, and ORP of the clay samples representing the depositional environment at the time of deposition and the current ground environment were measured and compared to investigate the changes in the post-depositional environment of the Quaternary clays in the Saga Plain.
Salt leaching of the Ariake clay
Compared with the Ariake clay deposited in the Saga Plain, the clay in the seabed of Isahaya Bay (Fig. 1) is much younger. In this study, the Ariake clay from the Saga Plain was designated as aged Ariake clay, and the clay from the seabed of Isahaya Bay was designated as young Ariake clay. It was assumed that the NaCl concentration of the young Ariake clay represented the initial depositional environment of the Ariake clay in the Saga Plain, and that the NaCl concentration of the aged Ariake clay represented the current ground environment of the Ariake clay in the Saga Plain. Therefore, the changes in the post-depositional environment of the Ariake clay in the Saga Plain could be investigated by comparing the NaCl concentrations between the young and aged Ariake clays.
Figure 12a shows the NaCl concentration distributions of the young Ariake clay samples at the eight locations. The NaCl concentrations ranged from 10 to 22 g/L, and the average value was approximately 16 g/L. The NaCl concentration did not change considerably with depth. Figure 12b shows the NaCl concentration distributions of the aged Ariake clay samples at the eight locations. The NaCl concentrations ranged from 0 to 15 g/L, and most of the values were less than 10 g/L. The distributions can be classified into three types. For the first type, the NaCl concentration increased linearly with depth at locations A-1, A-2, and A-3. For the second type, the NaCl concentration first increased and then decreased with depth at locations A-4, A-5, and A-6, i.e., the NaCl concentrations in the middle section were higher than those in the upper and lower sections, forming an arcuate distribution. For the third type, the NaCl concentrations were very low compared with those at other locations and were generally less than 2 g/L at locations A-7 and A-8. The comparison of the NaCl concentration distributions of the young and aged Ariake clays shown in Fig. 12 indicates that the NaCl concentration of the aged Ariake clay was lower than that of the young Ariake clay, i.e., salt leaching occurred in the Ariake clay in the Saga Plain. The main mechanism of salt leaching in Ariake clay is diffusion along with rainfall percolation and seepage flow from or to the Pleistocene gravelly sand layer (Chai et al. 2017). The salt leaching mechanism of the first type of distribution is the percolation of rainfall and diffusion of ions toward the ground surface. The salt leaching mechanism of the second type of distribution is the percolation of rainfall and diffusion of ions toward the ground surface, and advection and diffusion of ions toward the underlying sand layer. The salt leaching mechanism of the third type is the same as that of the second type with a higher seepage velocity.
The undisturbed and remolded su values of the aged Ariake clay at the eight locations were measured to investigate the effect of salt leaching on su and the strength sensitivity of the Ariake clay in the Saga Plain. No connection between the undisturbed su and the NaCl concentration was found. There was some correlation between the remolded su and the NaCl concentration, e.g., the NaCl concentrations (< 2 g/L) and the remolded su (< 0.5 kPa) values were both very low. Figure 13 shows the relationship between the strength sensitivity and the NaCl concentration for all the tested Ariake clay samples. The NaCl concentrations of the Ariake clay samples at locations A-7 and A-8 were very low (less than approximately 2 g/L) compared with those at the other locations, and the strength sensitivity values at locations A-7 and A-8 were much higher than those at the other locations. Since a small difference in the other features of the compared samples will not result in a significant difference of their remolded su, it was considered that the strength sensitivity of the Ariake clay might increase significantly when the NaCl concentration was less than 2 g/L due to the significant decrease in the remolded su. However, the strength sensitivity did not increase significantly at some of the points shown in Fig. 13, even when the NaCl concentrations were less than 2 g/L. The decrease in the remolded su was caused by the decrease in the liquid limit due to salt leaching. However, some researchers reported that salt leaching resulted in an increased liquid limit and remolded su. The relationship between salt leaching and the liquid limits of different clay minerals with different activities was investigated in this study.
The liquid limits of low-activity clay (kaolin clay) representing Ariake clay samples with low-activity clay minerals and high-activity clay (montmorillonite clay) representing Ariake clay samples with high-activity clay minerals with different pore water NaCl concentrations are shown in Fig. 14. With a decrease in the NaCl concentration (salt leaching), the liquid limit of the low-activity clay decreased, whereas the liquid limit of the high-activity clay increased. Salt leaching (a decrease in the cation concentration) results in an increase in the thickness of the electric diffusive double layer (DDL) and an increase in the repulsive force between particles. Salt leaching reduced the frictional resistance of the many direct interparticle contacts present in the low-activity clay, consequently altering the soil microstructure and reducing the interparticle spacing. The decrease in interparticle spacing resulted in a decrease in the liquid limit. For high-activity clay, there were few direct interparticle contacts, and salt leaching increased the thickness of the DDL, thus increasing the interparticle spacing. The increase in interparticle spacing resulted in an increase in the liquid limit.
Only a decrease in the liquid limit resulted in an increase in the liquidity index and subsequently, a decrease in the remolded su and an increase in strength sensitivity. Therefore, salt leaching resulted in a higher strength sensitivity for only the low-activity clay, and the strength sensitivity of the high-activity clay did not change considerably. It has been suggested that when the NaCl concentration is less than approximately 2 g/L, the strength sensitivity of the Ariake clay with low-activity clay minerals will increase significantly due to the decrease in the liquid limit and remolded su. Therefore, salt leaching is probably a main cause of the high strength sensitivity of the Quaternary clays in the Saga Plain.
Ground environment change
It was assumed that the pH and ORP of the soil samples from the seabed of Isahaya Bay represented the initial depositional environment, while the pH and ORP of the soil samples from the Saga Plain represented the post-depositional environment. Therefore, the changes in the post-depositional environment of the clays in the Saga Plain could be investigated by comparing the pH and ORP results between the soil samples from the seabed of Isahaya Bay and the Saga Plain.
Figure 15 a and b shows the pH distributions of the soil samples collected from the seabed of Isahaya Bay and the Saga Plain, respectively. The pH values of the soil samples from the seabed of Isahaya Bay were greater than 7 (alkaline environment), and they were in a narrow range, while the pH values of the soil samples from the Saga Plain varied over a wide range and were sometimes less than 7 (acidic environment). The decreasing pH at certain depths, shown in Fig. 15b, might be due to the inorganic acids produced by the oxidation of minerals in the soil samples. Figure 16a and b shows the ORP distributions of the soil samples from the seabed of Isahaya Bay and the Saga Plain. Most of the ORP values of the soil samples from the seabed of Isahaya Bay were negative, which means that the initial depositional environment was a reducing environment. However, some ORP values of the soil samples from the Saga Plain were positive, which means that the current ground environments in some locations were oxidative. A comparison of the pH and ORP distributions of the soil samples from the seabed of Isahaya Bay and the Saga Plain indicated that the ground environment in the Saga Plain changed after deposition of the Quaternary deposits. The ground environment at some locations changed from an alkaline, reducing environment, to an acidic, oxidative environment.
The pH values of the soil samples from location A-8 in the Saga Plain were measured immediately after drilling, 106 days after drilling, and once more after the samples were treated with hydrogen peroxide (H2O2) to examine the oxidation potential of the soil. The pH values measured at these three times are compared in Fig. 17. The comparison results indicate that the soils became increasingly acidic with exposure to the air, with a potential to form a strongly acidic environment (pH ≈ 2), i.e., the ground environment in the Saga Plain might change to a strongly acidic environment in the presence of oxygen and water. One of the possible reasons for this change is that the biogenic pyrite (FeS2) within the Ariake clay formation was oxidized, and sulfuric acid was released when the ground environment changed to an oxidizing environment from a reducing environment. The oxidation of pyrites is described by the following equation:
Figure 18 shows a photo of the core samples collected from location A-8 from depths of 5–15 m. A rust-colored sand layer was present below the Ariake clay layer. Yellow or red soil indicates the presence of iron oxide (hematite, Fe2O3), which meant that the soil was weathered. The mechanism of weathering is the oxidation of pyrites, which generates iron oxide in the presence of oxygen and water. This kind of weathering changed the ground environment, resulting in low pH values and an acidic environment that influenced the engineering properties of the Quaternary clays. An alkaline environment is necessary for the development of strength in cement/lime-treated clay. The generation of sulfuric acid may have resulted in a reduction in the strength of the cement/lime-treated Ariake clay.
Conclusions
The engineering properties and the effect of the depositional environment and post-depositional processes on the engineering properties of the Quaternary clays in the Saga Plain, Japan, were investigated via laboratory testing of the core samples and undisturbed soil samples. Based on the test results, the following conclusions can be drawn.
- (1)
Engineering properties. The clays in the Saga Plain had a high void ratio, high water content, high compressibility, high sensitivity, and low stiffness. The water contents were mostly higher than their corresponding liquid limits. The one-dimensional compression curves plotted above the SCL proposed by Burland. The compression index was still high for clays deeper than 20 m below the ground surface. The clays had high strength sensitivity and stress sensitivity. The values of E50/su varied from approximately 70 to 260. The values of the strength sensitivity are high, even for the young sediment from the seabed of Isahaya Bay. These engineering properties indicate that the Quaternary clays in the Saga Plain underwent some degree of cementation and have strong structures.
- (2)
Effect of the depositional environment. During the Quaternary period, four large volcanic eruptions occurred around the Saga Plain. The Quaternary clays in the Saga Plain are the deposits of fine soil particles eroded and transported from the watershed, which was widely covered with pyroclastic materials. Although it is challenging to quantify the effect of pyroclastic ash on the compressibility and strength properties of clays, it was considered that the deposition of volcanic ash within the clay deposits resulted in a certain degree of cementation of the clays, and this was the main driver of the strong structure of the clays in the Saga Plain.
- (3)
Effect of the post-depositional processes. When the NaCl concentration was less than approximately 2 g/L, the strength sensitivity of the Ariake clay samples with low-activity clay minerals increased significantly due to the decrease in the liquid limit and subsequently significant decrease in remolded su. Therefore, salt leaching in the Ariake clay formation and the cementation effect of the volcanic ash were likely the main causes for the high strength sensitivity of the clays in the Saga Plain. The ground environment at some locations changed from an alkaline, reducing environment, to an acidic, oxidative environment. The ground environment has the potential to become a strongly acidic environment due to oxidation of pyrites, and this may deteriorate the strength of the cement/lime-treated clays in the Saga Plain.
References
Arulrajah A, Nikraz H, Bo MW (2005) In-situ testing of Singapore marine clay at Changi. Geotech Geol Eng 23(2):111–130
Bjerrum L (1967) Engineering geology of Norwegian normally consolidated marine clays as related to settlements of buildings. Geotechnique 17:81–118
Brand EW, Brenner RP (1981) Soft clay engineering. Elsevier, Amsterdam
Burland JB (1990) On the compressibility and shear strength of natural clays. Géotechnique 40(3):329–378
Cecconi M, Scarapazzi G, Viggiani GMB (2010) On the geology and the geotechnical properties of pyroclastic flow deposits of the Colli Albani. Bull Eng Geol Environ 69:185–206
Chai JC, Hino T, Shen SL, Carter JP (2017) Holocene deposits in Saga Plain: leaching mechanism and soil sensitivity. Geotech Geol Eng 35(5):2107–2122
Chandler RJ (2000) Clay sediments in depositional basin: the geotechnical cycle. Q J Eng Geol Hydrol 33(1):7–39
Chung CK, Finno RJ (1992) Influence of depositional processes on the geotechnical parameters of Chicago glacial clays. Eng Geol 32(4):225–242
Cohen KM, Finney SC, Gibbard PL, Fan JX (2013) The ICS international chronostratigraphic chart. Episodes 36(3):199–204
Du YJ, Hayashi S, Shen SL (2014) Impact of laver treatment practices on the geoenvironmental properties of sediments in the Ariake Sea. Mar Pollut Bull 81(1):41–48
Graham J, Shields DH (1985) Influence of geology and geological processes on the geotechnical properties of a plastic clay. Eng Geol 22:109–126
He P, Ohtsubo M, Abe H, Higasgi T, Kanayama M (2014) Quick clay development and cation composition of pore water in marine sediments from the Ariake Bay Area, Japan. Int J Geosci 5:595–606
Hino T, Jia R, Sueyoshi S, Harianto T (2012) Effect of environment change on the strength of cement/lime treated clays. Front Struct Civ Eng 6(2):153–165
Hino T, Chai JC, Negami T, Bergado DT, Jia R (2014) Assessment of the effects of sea-level change on the geoenvironment: the case of the Ariake Sea coastal lowlands. In: Proceedings of the 9th international symposium on lowland technology, Saga, Japan, pp 21–30
Hong ZS (2006) Correlating compression properties of sensitive clays using void index. Géotechnique 56(8):573–577
Hong ZS, Tsuchida T (1999) On compression characteristics of Ariake clays. Can Geotech J 36:807–814
Horpibulsuk S, Shibuya S, Fuenkajorn K, Katkan W (2007) Assessment of engineering properties of Bangkok clay. Can Geotech J 44(2):173–187
Horpibulsuk S, Yangsukaseam N, Chinkulkijniwat A, Du YJ (2011) Compressibility and permeability of Bangkok clay compared with kaolinite and bentonite. Appl Clay Sci 52:150–159
Howayek AE, Bobet A, Santagata M (2017) Geologic origin effects on mineralogy, index properties and fabric of a fine-grained carbonatic deposit. Eng Geol 216:108–121
Ismael N (1993) Laboratory and field leaching tests on coastal salt-bearing soils. J Geotech Eng 119(3):453–470
Kim YT, Do TH (2011) Experimental evaluation of leaching effects on the compressibility of marine clay and its strain rate dependency. Mar Georesour Geotechnol 29(1):16–29
Liu SY, Shao GH, Du YJ, Cai GJ (2011) Depositional and geotechnical properties of marine clays in Lianyungang, China. Eng Geol 121:66–74
Liu MD, Zhuang ZL, Horpibulsuk S (2013) Estimation of the compression behaviour of reconstituted clays. Eng Geol 167:84–94
Matsui K, Kurobe T, Kato Y (1963) Pedological problems concerning volcanic ash in Japan. Quat Res 3(1-2):40–58 (in Japanese)
Mitchell JK, Soga K (2005) Fundamentals of soil behavior. Wiley, New York
Miura N, Chai JC, Hino T, Shimoyama S (1998) Depositional environment and geotechnical properties of soft deposit in Saga Plain. Indian Geotech J 28(2):121–146
Mizota C, Longstaffe FJ (1996) Detrital origin for smectite in soils and sediments from the coastal plain of Ariake Bay, northern Kyushu, Japan. Geoderma 73(1-2):125–130
Moore JG, Brown JD, Rashid MA (1977) The effect of leaching on engineering behaviour of a marine sediment. Géotechnique 27(4):517–531
Nguyen XP, Cui YJ, Tang AM, Deng YF, Li XL, Wouters L (2013) Effects of pore water chemical composition on the hydro-mechanical behavior of natural stiff clays. Eng Geol 166:52–64
Ohtsubo M, Egashira K, Kashima K (1995) Depositional and post-depositional geochemistry and its correlation with geotechnical properties of the marine clays in Ariake Bay. Geotechnique 45:509–523
Pineda JA, Suwal LP, Kelly RB, Bates L, Sloan SW (2016) Characterisation of Ballina clay. Géotechnique 66(7):556–577
Quigley (1980) Geology, mineralogy and geochemistry of Canadian soft soils: geotechnical perspective. Can Geotech J 17:261–285
Shen SL, Wu HN, Cui YJ, Yin ZY (2014) Long-term settlement behaviour of metro tunnels in the soft deposits of Shanghai. Tunneling Undergr Space Technol 40:309–323
Shimoyama S, Matsuura H, Hino T (2010) Geology of the Saga District, Quadrangle Series, 1:50,000, Fukuoka (14), No. 71, NI-52-11-9, 97 (in Japanese)
Sridharan A, Prakash K (1999) Influence of clay mineralogy and pore-medium chemistry on clay sediment formation. Can Geotech J 36:961–966
Torrance JK, Ohtsubo M (1995) Ariake bay quick clays: a comparison with the general model. Soils Found 35(1):11–19
Warkentin BP, Yong RN (1962) Shear strength of montmorillonite and kaolinite related to interparticle forces. Clay Miner 9:210–218
Woo SM, Moh ZC (1977) Effect of leaching on undrained shear strength behavior of a sedimented clay. Proc. IX Int. Conf. on Soil Mech. and Found. Engrg., Tokyo, Japan, pp 51–464
Wu CJ, Ye GL, Zhang LL, Bishop D, Wang JH (2015) Depositional environment and geotechnical properties of Shanghai clay: a comparison with Ariake and Bangkok clays. Bull Eng Geol Environ 74:717–732
Xu YS, Shen SL, Du YJ (2009) Geological and hydrogeological environment in Shanghai with geohazards to construction and maintenance of infrastructures. Environ Geol 109:241–254
Ye GL, Lin N, Bao XH, Gu LL, Yadav SK (2018) Effect of Quaternary transgression and regression on the engineering properties of Shanghai soft clays. Eng Geol 239:321–329
Zhou GY, Esaki T, Mori J (2003) GIS-based spatial and temporal prediction system development for regional land subsidence hazard mitigation. Environ Geol 44:665–678
Acknowledgments
The site investigation data reported in this paper are from the Ariake Sea Coastal Road Development Office, Saga Prefecture, Japan, whose generosity is greatly appreciated.
Funding
The authors would like to acknowledge the financial support of the National Key Research and Development Program of China (Grant No. 2017YFC0805402), the Major Program of National Natural Science Foundation of China (Grant No. 51890911), and the National Natural Science Foundation of China (Grant No. 51509181).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, R., Zheng, G., Lei, H. et al. The effects of the depositional environment and post-depositional processes on the engineering properties of Quaternary clays in the Saga Plain. Bull Eng Geol Environ 79, 1137–1152 (2020). https://doi.org/10.1007/s10064-019-01653-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10064-019-01653-5