Abstract
Purpose
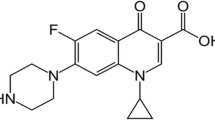
Chromium species in higher oxidation states are toxic and carcinogenic. But lower oxidation state as Cr(III) microelement shows potential antimicrobial properties. Cefoperazone sodium is a parenteral bactericidal third-generation cephalosporin antibiotic effective in treating Pseudomonas bacterial infections. A new drug formed with the complexation of metal and drug. Hence, newer coordinated drugs were explored that can also show the antimicrobial effectiveness similar to free drug.
Methods
cis-[Cr(C2O4)2(H2O)2]− was synthesized by the proposed method and its complexation with cefoperazone was done by reflux for 5–6 h with stirring. The structure was characterized by spectroscopic techniques (UV, IR, mass, and AAS). The antimicrobial study was done using agar disk-diffusion method. The interaction of the ligand substitution reaction between cis-[Cr(C2O4)2(H2O)2]− and cefoperazone (CFZ) has been investigated in aqueous medium. The reaction has been found to proceed by depending on ligand concentration.
Results
The kinetic reaction was studied at [CFZ] varied from 1.50 × 10−2 mol dm−3 to 4.50 × 10−2 mol dm−3 with pH variation from 3.0 to 5.0. The rate of reaction is found to increase in [H+] and [CFZ]. The activation parameters ΔH# and ΔS# are found to be 42.72 kJ mol−1 and − 182.41 J K−1 mol−1 respectively. The positive values of activation enthalpy (ΔH#) shows that the decomposition process is endothermic.
Conclusion
Compared to the free cefoperazone, complexation with chromium(III) metal ion shows similar antimicrobial activity. The reaction takes place through an associative interchange mechanism.









Similar content being viewed by others
References
Vincent JB. The bioinorganic chemistry of chromium (III). Polyhedron. 2001;20:1–26.
Yamamoto A, Wada O, Ono T. Isolation of a biologically active low-molecular-mass chromium compound from rabbit liver. Eur J Biochem. 1987;165:627–31.
Szablowicz M, Kita E. New chromium(III)-nicotinate complexes. Kinetics and mechanism of nicotinate ligand liberation in acidic media. Transit Met Chem. 2005;30:623–9.
Behera B, Behera J. Drug metal ion interaction: kinetics and mechanism of interaction of cis-bis (oxalate)diaquochromium(III) ion with ampicillin in aqueous medium. Chem Sci Trans. 2017;6(4):535–44.
Baral D, Rout S, Behera J, Si S, Mohanty P. Kinetics and mechanism of interaction of hexaaquachromium (III) with L-dopa in aqueous medium: comparative antiparkinsonian studies. Transit Met Chem. 2011;36:231–6.
Zavitsanos K, Petrou AL. Kinetics and mechanisms of the chromium(III) reactions with 2,4- and 2,5-dihydroxybenzoic acids in weak acidic aqueous solutions. Bioinorg Chem Appl. 2010;2010:1–10.
Thoma V, Tampouris K, Petrou AL. Kinetics and mechanism of the reaction between chromium(III) and 3,4-dihydroxy-phenyl-propenoic acid (caffeic acid) in weak acidic aqueous solutions. Bioinorg Chem Appl. 2008;2008:1–7.
Messih MF. Kinetics and mechanism of interaction between chromium(III) and ethylenediaminetetra-3-propinate in aqueous acidic media. Adv Chem Eng Sci. 2013;3:98–104.
Sorenson JRJ. Copper chelates as possible active forms of the antiarthritic agents. J Med Chem. 1976;19:135–41.
Brown DH, Smith WE, Teape JW. Antiinflammatory effects of some copper complexes. J Med Chem. 1980;23:729–33.
Bergan T. Pharmacokinetic properties of the cephalosporins. Drugs. 1987;34:89–104.
Cunha BA. Third-generation cephalosporins: a review. Clin Ther. 1982;14:616–52.
Chin NX, Gu JW, Fang W, Neu HC. In vitro activity and beta-lactamase stability of GR69153, a new long-acting cephalosporin. Antimicrob Agents Chemother. 1991;35:259–66.
Fu KP, Foleno BD, Lafredo SC, LoCoco JM, Isaacson DM. In vitro and in vivo antibacterial activities of FK037, a novel parenteral broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1993;37:301–7.
Parfitt K. The complete drug reference: a Martindale. 33rd ed. London: The Pharmaceutical Press; 2002.
Kalman D, Barriere SL. Review of the pharmacology, pharmacokinetics, and clinical use of cephalosporins. Tex Heart Inst J. 1990;17:203–15.
Al-Delaimy WK, Willet WC, Manson JE, Speizer FE, Hu FB. Smoking and mortality among women with type 2 diabetes: the Nurses’ Health Study cohort. Diabetes Care. 2001;24:2043–8.
Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol. 2002;155:387–93.
Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease: a subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614–20.
Islam MS, Farooque MA, Bodruddoza MAK, Mosaddik MA, Alam MS. Antimirobial and toxicological studies of mixed ligand transition metal complexes of Schiff bases. J Biol Sci. 2002;2:797–9.
Singh DP, Kumar R, Malik V, Tyagi P. Template synthesis, spectroscopic studies and biological activities of macrocyclic complexes derived from thiocarbohydrazide and glyoxal. J Enzyme Inhib Med Chem. 2007;22:177–82.
Sakagami N, Kita E, Kita P, Winsniewska J, Kaizaki S. The crystal structures of bis(oxalato)chromium(III) complexes with histamine and B6 vitamin: Na[Cr(ox)2(hm)]·3H2O and Na[Cr(ox)2(PM)]·H2O (hm, histamine; PM, pyridoxamine). Polyhedron. 1999;18:2001–7.
Anacona JR, Bravo A, Lopez ME. Antibacterial activity of cefoperazone metal complexes. Lt Am J Pharm. 2012;31:27–31.
Dash SC, Das NN, Mohanty P. Kinetics and mechanism of the reaction of ranitidine hydrochloride with trans-(diaqua) (N,N’-ethylene-bis-salicylamide) chromium(III) and hexa-aquachromium(III) ion – a comparative study. Indian J Chem Technol. 2011;18:132–6.
Debnath A, Hussain F, Masram DT. Synthesis, characterisation, and antifungal studies of Cr(III) complex of norfloxacin and bipiridyl ligand. Bioinorg Chem Appl. 2014;2014:1–7.
Anacona JR, Gil CC. Synthesis and antibacterial activity of cefoxitin metal complexes. Transit Met Chem. 2005;30:605–9.
Anacona JR, Acosta F. Synthesis and antibacterial activity of cephradine metal complexes. J Coord Chem. 2006;59:621–7.
Brauer G. Handbook of preparative inorganic chemistry. 2nd ed. Newyork, London: Academic Press; 1965. vol 2. pp. 1372.
Schenk C, Stieger H, Kelm H. Kinetik und Mechanismen der Bildungsreaktionen der Aquo-Oxalato-Chrom(III)-Komplexionen in wäßrigen Lösungen. Z Anorg Allg Chem. 1972;391:1–10.
Frost AA, Peasron RG. Kinetics and mechanism. New York: Wiley; 1961.
Franchini GC, Giusti A, Preti C, Tosi L, Zannini P. Cefoperazone (Hcefopz) interacts with transition metal(II) ions to give [M(cefopz)Cl] complexes (M=Fe, Co, Ni, Cu and Cd) and [Fe(cefopz)Cl]Cl which were characterized by physicochemical and spectroscopic methods. Polyhedron. 1985;9:1553–8.
Hadjikostas CC, Katsoulos GA, Shakhatreh SK. Synthesis and spectral studies of some new palladium(II) and platinum(II) dithiocarbimato complexes. Reactions of bases with the corresponding N alkyldithiocarbamates. Inorg Chim Acta. 1987;133:129–32.
Castillo M, Criado JJ, Macias B, Vaquero MV. Chemistry of dithio-carbamate derivatives of amino acids. I. Study of some dithiocarbamate derivatives of linear α-amino acids and their nickel(II) complexes. Inorg Chim Acta. 1986;124:127–32.
Lutfullah SS, Rahman N, Azmi SNH, Iqbal B, Amburk MIBB, Bait MIB, et al. UV spectrophotometric determination of Cu(II) in synthetic mixture and water samples. J Chin Chem Soc. 2010;57:622–31.
Mashaly MM, Abd-Elwahab ZH, Faheim AA. Preparation, spectral characterization and antimicrobial activities of Schiff base complexes derived from 4-aminoantipyrine. Mixed ligand complexes with 2-aminopyridine, 8-hydroxyquinoline and oxalic acid and their pyrolytical products. J Chin Chem Soc. 2004;51:901–15.
Barnes DJ, Chapman RL, Stephens FS, Vagg RS. Studies on the metal amide bond. VII. Metal complexes of the flexible N4 ligand N,N′-bis(2′-pyridinecarboxamide)1,2-ethane. Inorg Chim Acta. 1981;51:155–62.
Garg BS, Bhojak N, Dwivedi P, Kumar V. Copper(II) complexes of acid amide derivatives of 2-aminopyridine and an exogenous ligand. Transit Met Chem. 1999;24:463–6.
Patel AK, Patel JC, Dholariya HR, Patel KS, Patel VK, Patel KD. Synthesis, characterization and biological evaluation: copper(II) complexes of hydroxy coumarins with ciprofloxacin research. Res J Pharm, Bio And Chem Sci. 2013;4:564–79.
Anacona JR, Osorio I. Synthesis and antibacterial activity of copper(II) complexes with sulphathiazole and cephalosporin ligands. Transit Met Chem. 2008;33:517–21.
Patel KS, Patel JC, Dholariya HR, Patel VK, Patel KD. Synthesis of Cu(II), Ni(II), Co(II), and Mn(II) Complexes with ciprofloxacin and their evaluation of antimicrobial, antioxidant and anti-tubercular activity. O. J. Metal. 2012;2:49–59.
Bootwala S, Tariq M, Somasundaran S, Aruna K. Synthesis, spectroscopic and biological characterization of some transition metal complexes with ethyl 2-{[(2e, 3 Z)-4-hydroxypent-3-en-2-ylidene] amino}-4, 5, 6, 7-tetrahydro-1-benzothiophene-3 carboxylate. Int J Pharm Bio Sci. 2013;3:345–54.
Alekseev VG. Drug synthesis methods and production technologies metal complexes of penicillins and cephalosporins (review). Pharm Chem J. 2012;45:679–97.
Obeidat M, Shatnawi M, Al-alawi M, Al-Zu’bi E, Al-Dmoor H, Al-Qudah M, et al. Antimicrobial activity of crude extracts of some plant leaves. Res J Microbiol. 2012;7:59–67.
Shungu DL, Weinberg E, Gadebusch HH. Tentative interpretive standards for disk diffusion susceptibility testing with norfloxacin (MK-0366, AM-75). Antimicrob Agents Chemother. 1983;23:256–60.
Acknowledgements
The authors thank UGC, Government of India, New Delhi for providing UGC-BSR (Basic Science Research) Fellowship. We are grateful to Central Drug Research Institute, Lucknow for supporting CHN and mass analysis, and Sri Vasavi Institute of Pharmaceutical Sciences, Tadepaligudem, Andhra Pradesh for Antimicrobial study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Behera, J., Behera, B. Kinetics and Mechanism of Interaction of cis-Diaquabis(oxalato)chromate(III) with Cefoperazone in Aqueous Medium: as an Antibacterial Study. J Pharm Innov 15, 41–50 (2020). https://doi.org/10.1007/s12247-018-9368-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9368-3